It was on January 28, 2025 that the news of the first reported death probably due to Guillain-Barré Syndrome (GBS) in Maharashtra. However, the most shocking thing was the fact that there were more than 100 cases in Pune! Can you believe it? With the rise in the number of paralysis-related deaths in hospitals, people are now starting to question the safety of vaccines, especially now that there is the possibility of the same causing the death of the tribal girls somewhere in 2009. Moreover, people are investigating quite thoroughly whether health authorities are reacting to this worrying situation at all, or maybe such a situation does not exist.
So, let’s get to the bottom of the problem. The behind-the-scenes facts of those alarming events are the ones that we must necessarily be aware of and, in addition, share our knowledge with others. For sure, there are numerous researches that suggest occurrence of Guillain-Barré syndrome following vaccination is rare, but to be honest, we can’t really overlook the fact that the number of people becoming more and more concerned is growing. It’s a hard one, and it’s certainly one of the topics we are obliged to discuss.

https://qvive.in/india-news/trial-and-error-ethical-violations-of-hpv-vaccination-trials-in-india-noxious-vaccines-by-bill-gates/
A lot of people have been reading about Guillain-Barré Syndrome lately. They came to know that it is a condition that attacks the nervous system and is not a rare event that occurs only after a person is vaccinated. This is when the body’s immune system mistakenly invades the nerves leading to paralysis and, in some rare cases, death. One who has got GBS may have different degrees of paralysis subsequent to receiving a vaccine.
Despite Proven Safety of HPV Vaccines, More Parents Have Concerns:
An ethical violations surrounding HPV vaccination trials in India, particularly focusing on a project in Khammam district where 14,000 tribal girls aged 10-14 were vaccinated with Gardasil. The project, funded by the Gates Foundation and conducted by PATH, faced backlash after several girls died and many reported adverse effects. Critics argue that consent was improperly obtained, often from hostel wardens instead of parents, and that the girls were misled about the vaccine’s safety and efficacy. The Indian government later acknowledged that ethical guidelines were violated during the trial. The situation raises questions about the role of the government in vaccine development and the trustworthiness of health initiatives, especially in vulnerable communities.
The article also highlights broader concerns regarding vaccine safety and the influence of pharmaceutical companies in public health policies.
Who are the petitioners in the HPV proceedings before the Indian Supreme Court?
Petitioner 1: Kalpana Mehta, founding member of Saheli Women’s Resource Centre. For 30 years, she has been active in the women’s health movement. She worked on campaigns against hazardous contraceptives and for a women-centric approach to contraception. For the past years she has been with the organization Manasi Swasthya Sansthan in Indore to provid equality health care to economically disadvantaged women.
Petitioner 2: Nalini Bhanot, Voluntary Health Association of India in the area of public health. She has authored Taking Sides, a book on primary health care issues and has worked on child rights and various other initiatives relating to PDS, an acute Iodine deficiency.
Petitioner 3: V Rukmini Rao from the Gramya Resource Centre for Women. The Centre’s vision is to create a just society, which will provide more political, social and economic opportunities for women. They especially focus on tribal and dalit women, to help them achieve their rights, improve their lives and realize their potential. They work closely with community-based organizations, women’s organizations, youth leadership, and like-minded civil society organizations to seek justice for marginalized communities.
Which human rights were violated in the clinical trials case?
1. The right to informed consent
Valid informed consent requires the following elements: voluntarism, information disclosure and decision-making capacity. According to Article 7 of the International Covenant on Civil and Political Rights (ICCPR) medical experimentation without informed consent is considered “torture or cruel, inhuman or degrading treatment or punishment”. Moreover “[i]n particular, no one shall be subjected without his free consent to medical or scientific experimentation.”
2. The right to health
Article 12 of the International Covenant on Economic, Social and Cultural Rights (ICESCR) recognizes the right of everyone to enjoy the highest attainable standard of physical and mental health. A lack of monitoring during a clinical trial constitutes also a violation of this right to health, since monitoring is essential to identify injuries and respond promptly and adequately. Article 12.2(d) ICESCR requires States to take step towards the “creation of conditions which would assure to all medical services and medical attention in the event of sickness.” The creation of these conditions is hindered if a proper monitoring system is not set up.
3. The right to special care
In this case the trial subjects were entitled to special care. This right is entailed in three different international human rights covenants:
- The Convention on the Right of the Child (CRC). Article 24 (1) affirms the rights of the child to the enjoyment of the highest attainable standard of health.
- The Convention on the Elimination of All Form of Racial Discrimination (CERD). Article 5e (iv) obliges the States to guarantee the right of everyone in the enjoyment of the right to public health and medical care.
- The Convention to Eliminate All forms of Discrimination Against Women (CEDAW). Article 12(1) obliges the States to take “all appropriate measures to eliminate discrimination against women in the field of health care in order to ensure (..) access to health care services.”
Which laws are violated according to the petitioners?
According to the petitioners, the HPV vaccination project may have violated the Constitution of India. In particular, Article 21’s protection to life and personal liberty, as well as Articles 14 and 15 which provide for the right to equality and non-discrimination. The petitioners also allege violations of the International Convention on Economic, Social, and Cultural Rights (ICESCR) and the Convention for the Elimination of all forms of Discrimination Against Women (CEDAW), namely the right to the highest attainable standard of physical and mental health and to ensure that girls give informed consent and have access to adequate health care.
The inadequate informed consent process is further suggested to violate Indian regulations, because Schedule Y of the Drugs and Cosmetics Rules states:
“…All pediatric participants should be informed to the fullest extent possible about the study in a language and in terms they are able to understand. Where appropriate, pediatric participants should additionally assent to enroll in the study. Mature minors and adolescents should personally sign and date a separately designed written consent form…” (Paragraph 161 of the petition).
The petitioners also argue that the ICMR guidelines for biomedical research were not adhered to, even though they deal in detail with the informed consent process (Paragraph 193 of the petition):
“For all biomedical research involving human participants, the investigator must obtain the informed consent of the prospective participant or in the case of an individual who is not capable of giving informed consent, the consent of a legal guardian. Informed consent protects the individual’s freedom of choice and respect for individual’s autonomy and is given voluntarily to participate in research or not. Adequate information about the research is given in a simple and easily understandable unambiguous language in a document known as the Informed Consent Form with Participant/Patient Information Sheet.”
What do the petitioners demand?
A particular feature of public interest litigation is that the court can, for example, order government agencies to conduct certain investigations, provide guidelines for future action, or provide certain information. Thus, the petitioners demand:
- Access to information about the clinical trial and HPV vaccines in India, specifically information from PATH, the states of Andhra Pradesh & Gujarat, and the DCGI
- Order the Christian Medical College to do a fact-finding mission regarding the current health of those administered the vaccines
- Order to determine culpability of the CRO and the state governments
- Order to start criminal investigation
- Provide guidelines regarding civil and criminal liability for clinical trials in India
The Bill and Melinda Gates Foundation works in partnership with various organizations, including Gavi, the Vaccine Alliance, UNICEF, and the World Health Organization (WHO), to support HPV vaccine initiatives. Gavi has committed over $600 million from 2022 to accelerate the number of girls reached with the HPV vaccine and achieve the target of reaching 86 million adolescent girls by the end of 2025. The foundation also supports the development of new prophylactic HPV vaccines and tools to help countries better understand how vaccines might be used beyond current target populations.
Impact of Funding on HPV Vaccine Initiatives
The funding provided by the Bill and Melinda Gates Foundation has had a significant impact on HPV vaccine initiatives in low-income countries. In 2023, UNICEF is supplying HPV vaccines to 52 countries, with seven countries introducing the vaccine for the first time.The foundation’s support has also helped to increase access to HPV vaccines, particularly in countries with high burdens of cervical cancer.

It’s concerning that India has launched a new HPV vaccine named CERVAVAC, created by the Serum Institute of India with help from the Gates Foundation. This vaccine is designed to offer affordable protection against cervical cancer for many women and girls in India. The Indian government is also planning a nationwide vaccination program that matches global health goals set by groups like the World Health Organization (WHO).

HPV Vaccines Available in India
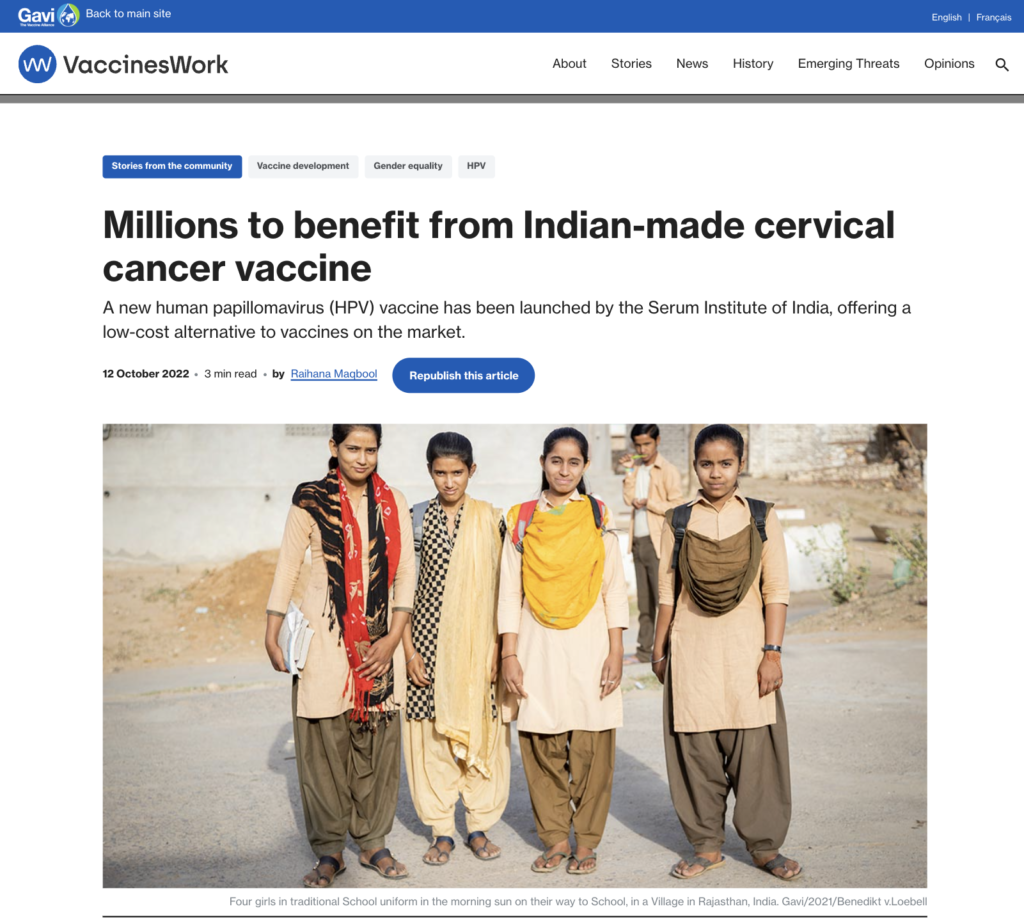
Types of HPV Vaccines Available in India
India currently has two main types of HPV vaccines available:
Here’s a more detailed look at the available vaccines:
- Cervarix (Bivalent): Targeted at HPV types 16 and 18.
- Gardasil (Quadrivalent): Provides protection against HPV types 6, 11, 16, and 18.
- Gardasil 9 (Nonavalent): Protects against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
- Cervavac (Quadrivalent): An Indian-made quadrivalent vaccine that targets the same HPV types as Gardasil.
The primary manufacturers of these HPV vaccines in India include:
- Merck & Co.: They produce Gardasil, which is marketed in India at a price that aims to make it accessible to a broader segment of the population.
- GlaxoSmithKline (GSK): They manufacture Cervarix, which is also available in the Indian market.
Additionally, the Serum Institute of India has developed an indigenously produced HPV vaccine named CERAVANAC qHPV, which was launched recently as a low-cost alternative aimed at improving accessibility for women in India. This vaccine is priced significantly lower than its counterparts from Merck and GSK, making it more affordable for the general population
Girls in government schools in Pune are getting HPV vaccinations to prevent cervical cancer. This program, backed by the Infosys Foundation, is happening in many cities like Chennai, Hyderabad, Bangalore, Mysore, Bhubaneshwar, and in Kerala. Various groups like the Rotary Club, Grace Cancer Foundation, and other hospitals in states are helping to make this happen.

Many people are worried about the HPV vaccine being given for free because it can have serious side effects that might even lead to death.
What Vaccines Are Known to Cause Neurological Conditions?
Several vaccines have been associated with rare cases of neurological conditions. While the overall risk is low, it is essential to be aware of these potential side effects. Some vaccines that have been linked to neurological conditions include:
Whole-cell pertussis-containing vaccines (DTaP, DTaP-IPV/Hib)
Measles, mumps, and rubella (MMR) vaccine
Human papillomavirus (HPV) vaccine
Hepatitis B vaccine
Tetanus-containing vaccines
Influenza vaccine
The benefits of vaccines in preventing serious diseases usually outweigh the potential risks of these rare neurological side effects. However, if you or a loved one experiences any concerning symptoms following vaccination, seek medical attention and consult with a vaccine injury lawyer to discuss your options.

Girls and Women – Gardasil Side effects
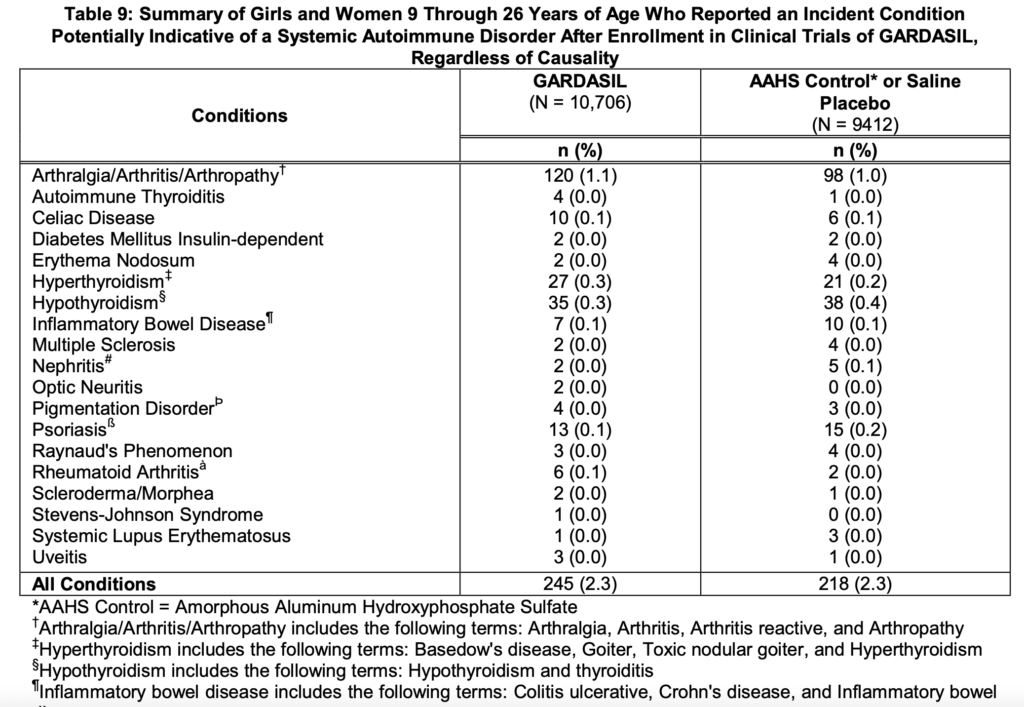
Boys and Men -Gardasil Side Effects
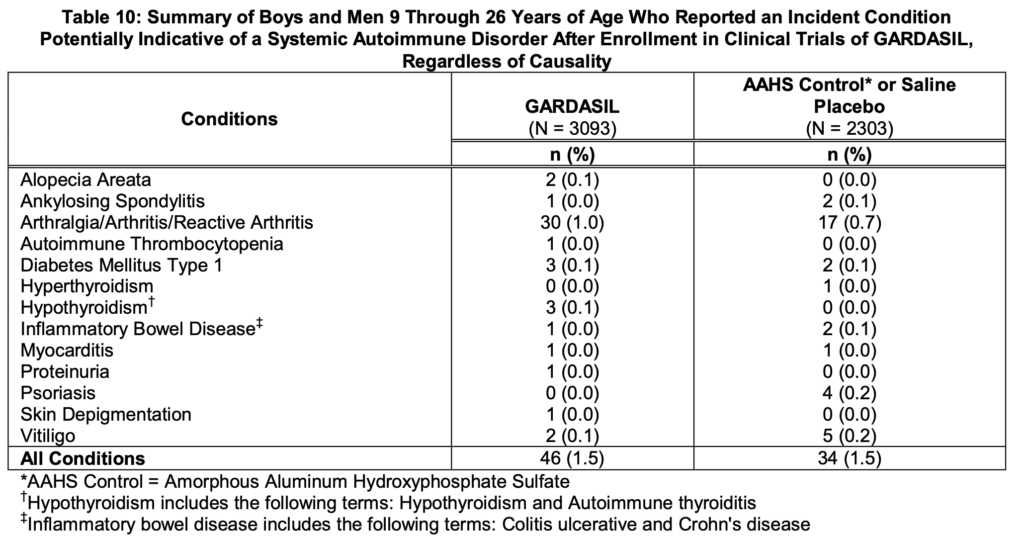
Cervarix Side Effects

Females 9 through 25 Years of Age – Cervarix Side Effects

Adverse events following HPV vaccination: 11 years of surveillance in Australia

Guillain-Barre Syndrome after HPV Vaccination: A Report from the Vaccine Adverse Event Reporting System (VAERS) (2006–2021) (S21.005)
Ref: https://www.neurology.org/doi/10.1212/WNL.98.18_supplement.3822
Myasthenia gravis following human papillomavirus vaccination: a case report
A 23-year-old woman with binocular vertical diplopia, bilateral ptosis (which worsened with left and down gazing), dysarthria, and dysphagia visited the outpatient department

Source: ECCHR, Facebook-Image
Also Read:
