COVID Deception
Are Vaccines Still Considered Safe? You will understand why governments and mainstream media persist in misleading the public and choose to remain silent about the connection between vaccines and deaths.
Before diving into this article, I suggest taking a look at the article linked below.
NOVAVAX = COVID Vaccine = COVOVAX = Nanoparticle Vaccine

Matrix-M is a vaccine adjuvant, a substance that is added to various vaccines to stimulate the immune response. It was patented in 2020 by Novavax.
Matrix‐M™, a proprietary saponin‐based Matrix adjuvant developed by Novavax, has been under investigation and development for over two decades and was most notably used in recent years as a key component of vaccines such as the NVX‐CoV2373 COVID‑19 vaccine. Early development work on ISCOM‐based and Matrix adjuvant technologies dates back to the 1980s–1990s, with further refinement into the Matrix‑M formulation emerging over the past 20 years. In clinical studies, Matrix‑M has been incorporated into several experimental and commercial vaccine candidates—including the widely reported NVX‑CoV2373 vaccine whose clinical trials began around 2020 and which received emergency or full authorization in 2021—demonstrating its long‐standing safety and immunostimulatory profile.
Matrix-M has been incorporated into several vaccines under development or already approved:
Combination Vaccines: Novavax is exploring its use in combined flu-COVID vaccines for broader protection.
COVID-19 Vaccine NVX-CoV2373 (Novavax): This recombinant protein-based vaccine uses Matrix-M to enhance its immunogenicity against SARS-CoV-2.
Malaria Vaccine R21/Matrix-M: Demonstrated high efficacy in clinical trials for malaria prevention.
Influenza Vaccines: Shown to provide cross-protection against multiple influenza strains.
Novavax, Inc. is an American biotechnology company based in Gaithersburg, Maryland, that develops vaccines to counter serious infectious diseases. Prior to 2020, company scientists developed experimental vaccines for influenza and respiratory syncytial virus (RSV), as well as Ebola and other emerging infectious diseases. During 2020, the company redirected its efforts to focus on the development and approval of its NVX-CoV2373 vaccine for COVID-19.
The COVID-19 vaccine Nuvaxovid was approved in the European Union at the end of 2021,and in Canada in February 2022, as the fifth vaccine against COVID-19, following Pfizer/BioNTech, Moderna, Janssen and AstraZeneca.
In June 2013, Novavax acquired the Matrix-M adjuvant platform with the purchase of Swedish company Isconova AB and renamed its new subsidiary Novavax AB.
NanoFlu™ Vaccine Description
Novavax, Inc.’s NanoFlu™ is a vaccine candidate that is a recombinant hemagglutinin (HA) protein nanoparticle influenza vaccine produced in an Sf9 insect cell-baculovirus system. NanoFlu uses HA protein amino acid sequences that are the same as the recommended wild-type virus HA sequences. NanoFlu contains Novavax’s patented saponin-based Matrix-M adjuvant, which is potent, well-tolerated, and stimulates high-quality and durable antibody responses and multifunctional CD4 and CD8 T-cell responses. Recombinant seasonal influenza vaccines have an essential advantage over other flu shots: once licensed for commercial sale, large quantities of vaccines can be manufactured cost-effectively without using the live influenza virus or eggs, says Novavax.
Novavax COVID-19 vaccine
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations.
Updated versions of the vaccine have been developed to provide coverage against the Omicron variant, with different formulas for 2023–2024 (containing a recombinant spike protein from lineage XBB.1.5) and 2024–2025 (containing recombinant spike protein from lineage JN.1).
Their strategy indicates that the JN.1 COVID variant will soon see a rise in cases.

CEPI was conceived in 2015 and formally launched in 2017 at the World Economic Forum (WEF) in Davos, Switzerland. It was co-founded and co-funded with US$460 million from the Bill and Melinda Gates Foundation, the Wellcome Trust, and the governments of India and Norway, and was later joined by the European Union (2019) and the United Kingdom (2020). CEPI is headquartered in Oslo, Norway.
CEPI has used “vaccine bonds” to “frontload” multi-year sovereign funding pledges. In 2019, the International Finance Facility for Immunisation (IFFIm) issued NOK 600 million in vaccine bonds to front-load the commitment by Norway, through Gavi, the Vaccine Alliance, to CEPI.
Here marks the beginning of the COVID-19 Agenda..
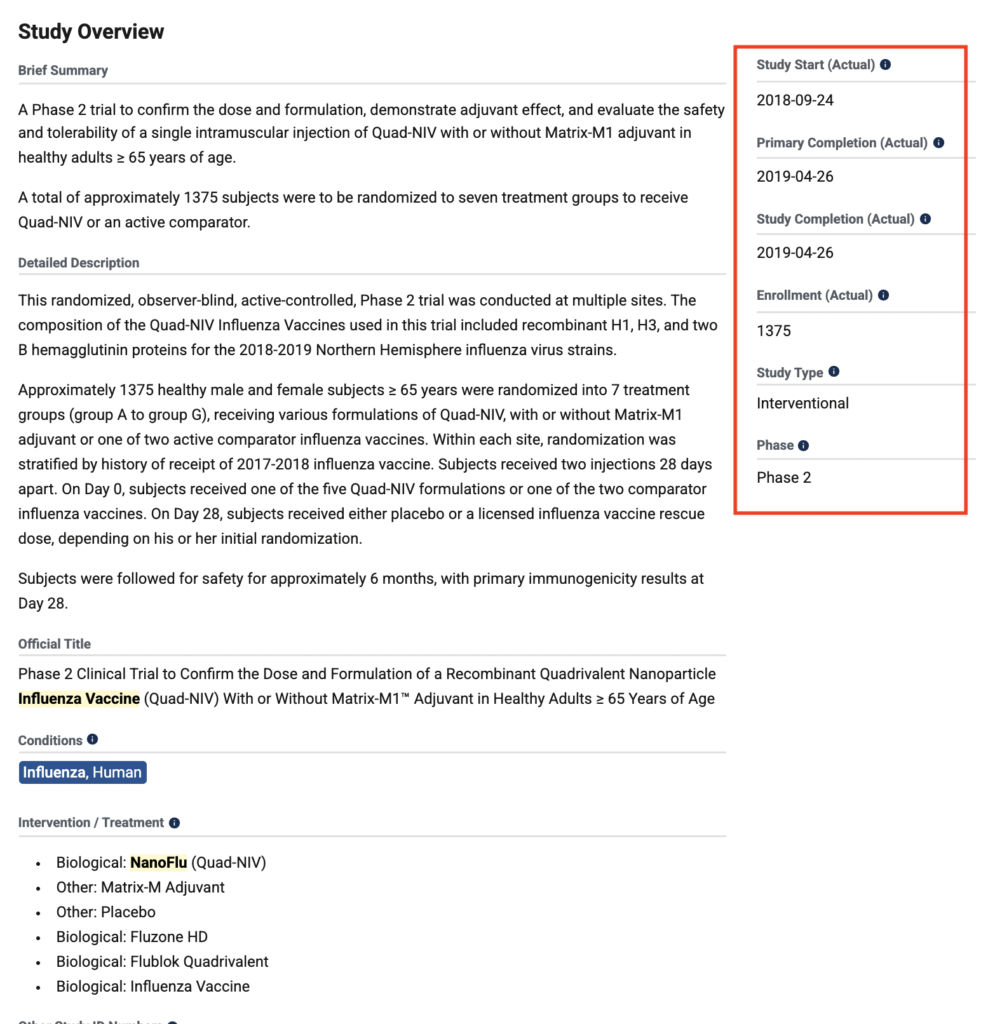
January 3, 2019: Novavax Announces Positive Phase 2 NanoFlu Results in Older Adults
Novavax NanoFlu is a recombinant hemagglutinin protein nanoparticle influenza vaccine candidate in a phase 2 clinical study.
(GLOBE NEWSWIRE) — Novavax, Inc. (NASDAQ: NVAX) today announced top-line results of its Phase 2 clinical trial of NanoFlu™. The trial compared the safety and immune responses of various quadrivalent formulations of NanoFlu, with or without Novavax’ Matrix-M™ adjuvant, with two U.S.-licensed influenza vaccines in 1,375 healthy adults 65 years of age and older.
Ref: https://ir.novavax.com/press-releases/2019-01-03-Novavax-Announces-Positive-Phase-2-NanoFlu-Results-in-Older-Adults
October 17, 2019 : NanoFlu Influenza Vaccine Phase 3 Study Launches
A Maryland based biotechnology company announced the initiation of a pivotal Phase 3 clinical trial for NanoFlu™, its recombinant quadrivalent seasonal influenza vaccine candidate, in adults aged 65 and over.
Ref: https://www.vax-before-travel.com/novavax-nanoflu-matrix-m-adjuvanted-recombinant-nanoparticle-vaccine-eliciting-antibodies-neutralize
Aug 04, 2020: Novavax Announces Positive Phase 1 Data for its COVID-19 Vaccine Candidate
- Phase 1 portion of the Phase 1/2 clinical trial evaluated two doses of Novavax’ COVID-19 vaccine across two dose levels (5 and 25 µg) in 131 healthy adults ages 18-59 years
Ref: https://web.archive.org/web/20210825075000/https://novavax.reportablenews.com/pr/novavax-announces-positive-phase-1-data-for-its-covid-19-vaccine-c

The depopulation agenda took shape following the announcement on October 15, 2019, when Novavax, Inc. revealed the start of a crucial Phase 3 clinical trial for NanoFlu. Then, on January 15, 2020, they declared that the U.S. Food and Drug Administration (FDA) had awarded Fast Track Designation to NanoFlu as a Covid Vaccine. Following this successful move, they began distributing it to the public, claiming it was safe while hiding significant side effects that contradicted other medications. The same flu vaccine was administered to older adults, leading to serious side effects, which sparked a Covid panic. They continued with the vaccine for three doses, but by the time the fourth dose was due, many people had started to see through the scam aimed at depopulation.
Following this, news emerges indicating that Novavax, a US-based biotech company, has collaborated with the Serum Institute of India to manufacture its COVID-19 vaccine, Covavax. The company asserts that the vaccine is 90% effective and safe for use.
The public should be told that vaccines may have long-term adverse effects

Are there serious side effects associated with the flu vaccine?
Serious side effects occur very rarely and are much lower than the risk of having severe complications from influenza. These include:
- Allergic reactions leading to rash, low blood pressure, facial swelling and breathlessness.
- Blood vessel and blood disorders such as lowered platelet levels and blood vessel inflammation, lymph node swelling.
- Nervous system disorders such as fits and progressive muscle weakness (Guillain-Barre Syndrome).

Video: Vaccines are Creating the Variants and Notable young people with thromboses
Prof. Montagnier referred to the vaccine program for the coronavirus as an “unacceptable mistake”. Mass vaccinations are a “scientific error as well as a medical error,” he said. “It is an unacceptable mistake. The history books will show that, because it is the vaccination that is creating the variants,” Prof. Luc Montagnier continued.
The prominent virologist explained that “there are antibodies, created by the vaccine,” forcing the virus to “find another solution” or die. This is where the variants are created. It is the variants that “are a production and result from the vaccination.”
NanoFlu Vaccine News
November 1, 2021 – NanoFlu™, its quadrivalent influenza nanoparticle vaccine, met all primary objectives in its pivotal Phase 3 clinical trial in older adults. Both vaccine candidates incorporate Novavax’s’ proprietary saponin-based Matrix-M™ adjuvant to enhance the immune response and stimulate high levels of neutralizing antibodies.
September 23, 2021 – “Despite high vaccination rates, limitations in the effectiveness of existing influenza vaccines leave significant disease burden unaddressed, particularly in older adults,” said Stanley C. Erck, President and Chief Executive Officer, Novavax. “These encouraging results reflect NanoFlu’s promise, especially as we currently have a combination COVID-19-influenza vaccine under evaluation for protection against two life-threatening diseases simultaneously.”
October 15, 2021 – Novavax, Inc. announced that Vivek Shinde, M.D., Vice President, Clinical Development, will deliver a presentation during the World Vaccine Congress Europe 2021. A topic of discussion will be Novavax’s COVID-NanoFlu™ Combination Vaccine, which combines the company’s recombinant nanoparticle protein-based COVID-19 and NanoFlu™ vaccine candidates with Matrix-M™ adjuvant in a single formulation.
September 23, 2021 – Novavax, Inc. announced complete results from a pivotal Phase 3 clinical trial of NanoFlu in The Lancet Infectious Diseases. In the thorough analysis, NanoFlu was well-tolerated and produced significantly enhanced humoral and cellular immune responses versus the comparator vaccine.
September 8, 2021 – Novavax, Inc. announced the enrollment of participants in a Phase 1/2 study to evaluate the safety and immunogenicity of a combination vaccine using Novavax’s NanoFlu seasonal influenza and COVID-19 vaccines. Both NVX-CoV2373 and NanoFlu have previously demonstrated strong results as standalone vaccines in Phase 3 clinical trials. In preclinical studies, the COVID-NanoFlu Combination Vaccine demonstrated robust, functional immune responses to each component of the quadrivalent influenza vaccine and the SARS-CoV-2 spike protein, with Matrix-M adjuvant playing a key role.
May 10, 2021 – Novavax, Inc. announced positive preclinical Data for Combination Influenza and COVID-19 Vaccine Candidate. The preclinical study found that the combination of NanoFlu/NVX-CoV2373 (qNIV/CoV2373) vaccine induced functional influenza and COVID antibodies in ferrets.
March 1, 2021 – Novavax, Inc. announced that they continue to advance the NanoFlu program, including exploring a combined NanoFlu/NVX-CoV2373 vaccine that could be used in a post-pandemic setting.
November 9, 2020 – Novavax, Inc. announced updates to its leadership team, including the appointment of Gregory F. Covino as Executive Vice President and Chief Financial Officer. Executive Vice President John Trizzino, who previously served as CFO, will become the Chief Commercial Officer while continuing as Chief Business Officer.
October 13, 2020 – Novavax, Inc. announced the formation of a leadership team to advance NanoFlu to regulatory licensure and the promotion of Russell (Rip) Wilson, J.D./M.B.A., to Executive Vice President and the newly-created role of NanoFlu™ General Manager. Mr. Wilson will focus exclusively on leading efforts to advance NanoFlu, the company’s influenza vaccine candidate, through global licensure, as well as the exploration of a combined influenza/COVID-19 vaccine that could be used in a post-pandemic setting. In addition, Novavax announced the results of its successful NanoFlu pivotal Phase 3 clinical trial earlier this year and intended to seek regulatory approval from the U.S. Food and Drug Administration under the accelerated approval pathway previously granted to the company.
January 15, 2020 – Novavax, Inc. announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track Designation for NanoFlu.
October 15, 2019 – Novavax, Inc. announced initiating a pivotal Phase 3 clinical trial for NanoFlu, its recombinant quadrivalent seasonal influenza vaccine candidate, in adults aged 65 and over.
August 5, 2019 – Novavax Reaches Agreement with the U.S. FDA on Pivotal Phase 3 Trial Design for NanoFlu.
January 03, 2019 – Novavax announced Positive Phase 2 NanoFlu Results in Older Adults.
September 19, 2017 – A Phase 1/2 clinical trial of our nanoparticle seasonal influenza vaccine candidate, including our proprietary Matrix-M adjuvant (“NanoFlu™”) in older adults.
Ref: https://www.vax-before-travel.com/vaccines/nanoflu-influenza-vaccine
The FDA is now saying that Nuvaxovid™ is the only COVID-19 vaccine in the U.S. that uses recombinant protein and isn’t mRNA, but it feels like they’re pushing the same agenda as in 2019.
Ref: https://ir.novavax.com/press-releases/2025-05-19-U-S-FDA-Approves-BLA-for-Novavaxs-COVID-19-Vaccine
June 5, 2023: Novavax Announces Agreement with Bill & Melinda Gates Medical Research Institute to Include Matrix-M™ Adjuvant as Potential Component in Vaccine Research
Ref: https://ir.novavax.com/press-releases/2023-06-05-Novavax-Announces-Agreement-with-Bill-Melinda-Gates-Medical-Research-Institute-to-Include-Matrix-M-TM-Adjuvant-as-Potential-Component-in-Vaccine-Research
7 Aug 2020: Novavax signs COVID-19 vaccine supply deal with India’s Serum Institute The deal will foster development and commercialisation of COVID-19 vaccine The Indian drugmaker will have exclusive rights for the vaccine in India The experimental vaccine is said to produce high levels of antibodies against COVID-19
The world’s largest vaccine manufacturer wants to conduct trials of Novavax’s COVID-19 vaccine in India. The Serum Institute of India has applied for government approval, hours after the US drugmaker announced its vaccine was nearly 90 per cent effective in a UK trial.
EMERGENCY USE AUTHORIZATION (EUA) OF THE NOVAVAX COVID-19 VACCINE, ADJUVANTED (2024 – 2025 FORMULA) TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) IN INDIVIDUALS 12 YEARS OF AGE AND OLDER
WHAT ARE THE RISKS OF THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
There is a remote chance that the vaccine could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
• Difficulty breathing
• Swelling of the face and throat
• A fast heartbeat
• A bad rash all over the body
• Dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received the vaccine. In most of these people, symptoms began within 10 days following vaccination. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine:
• Chest pain
• Shortness of breath
• Feelings of having a fast-beating, fluttering, or pounding heart
Side effects that have been reported in clinical trials with the Novavax COVID-19 Vaccine,
Adjuvanted include:
• Myocarditis (inflammation of the heart muscle)
• Pericarditis (inflammation of the lining outside the heart)
• Injection site reactions: pain/tenderness, swelling, redness, and itching
• General side effects: fatigue or generally feeling unwell, muscle pain, headache, joint pain,
nausea, vomiting, fever, chills
• Allergic reactions such as hives and swelling of the face
• Swollen lymph nodes
Side effects that have been reported in post-authorization use with the Novavax COVID-19
Vaccine, Adjuvanted include:
• Severe allergic reactions
• Myocarditis (inflammation of the heart muscle)
• Pericarditis (inflammation of the lining outside the heart)
August 2024 5
• Paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), hypoesthesia
(decreased feeling or sensitivity, especially in the skin)
Ref: https://www.fda.gov/media/159898/download?attachment
Clinical Considerations: Myocarditis and Pericarditis after Receipt of COVID-19 Vaccines Among Adolescents and Young Adults

WHAT ARE THE RISKS OF THE COVOVAX/COVID-19 Vaccine, Adjuvanted 2023-2024 Formula (Omicron XBB.1.5)?
Like all medicines, this vaccine can cause side effects, which can be life-threatening,
Side effects that have been reported with the COVOVAX/COVID-19 Vaccine, Adjuvanted 2023-2024 Formula (Omicron XBB.1.5) include:
Postmarketing Experience
The following adverse reactions have been identified during post-authorization use of the Novavax COVID-19 Vaccine.
Cardiac Disorders: myocarditis, pericarditis
Immune System Disorders: anaphylaxis
Nervous System Disorders: paresthesia, hypoesthesia, Guillain–Barré syndrome (GBS)
*Serious adverse events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that, based on appropriate medical judgment, may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
Instructions for Reporting to VAERS 2025
Vaccination providers should complete and submit a VAERS form to FDA using one of the following methods:
- Complete and submit the report online: HTTPS://VAERS.HHS.GOV/REPORTEVENT.HTML, or
- If you are unable to submit this form electronically, you may fax it to VAERS at 1-877-721-0366. If you need additional help submitting a report, you may call the VAERS toll-free information line at 1-800-822-7967 or send an email to info@vaers.org.
IMPORTANT: When reporting adverse events or vaccine administration errors to VAERS, please complete the entire form with detailed information. It is important that the information reported to the FDA be as detailed and as complete as possible. Information to include:
- Patient demographics (e.g., patient name, date of birth)
- Pertinent medical history
- Pertinent details regarding admission and course of illness
- Concomitant medications
- Timing of adverse event(s) in relationship to administration of the Novavax COVID-19 Vaccine, Adjuvanted (2024 – 2025 Formula)
- Pertinent laboratory and virology information
- Outcome of the event and any additional follow-up information if it is available at the time of the VAERS report. Subsequent reporting of follow-up information should be completed if additional details become available.
Ref: https://www.seruminstitute.com/pdf/COVOVAX_Fact_Sheet.pdf
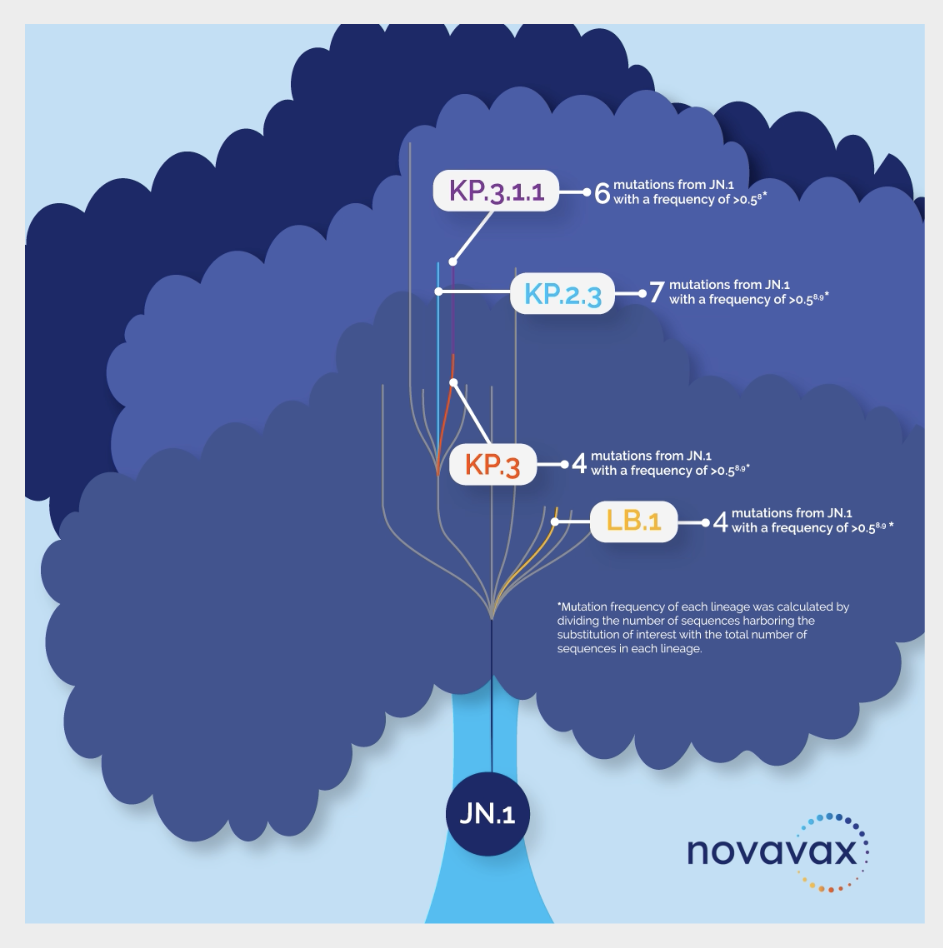
The virus that causes COVID-19 has continuously mutated, evolving from the Wuhan strain in 2020 to new strains that have included the Alpha, Beta, Gamma, Delta, Omicron variants and XBB subvariants. As of early fall 2024, the primary subvariants circulating in the United States are descendants of the JN.1 strain

Just days after President Trump announced that the US is pulling out of the World Health Organization. Italy’s choice to withdraw from the WHO has prompted many in India to ask why their government has not done the same. With the pressing global health issues after vaccine side effects and the WHO’s mishandling of the pandemics, there are calls within India for the government to take a similar stance.
Gavi, the Vaccine Alliance
Gavi serves as a co-convener of COVAX, which is the vaccine component of the Access to COVID-19 Tools (ACT) Accelerator, in collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI), the World Health Organization (WHO), and UNICEF.
Gavi works closely with its Alliance partners, including UNICEF and WHO, as well as various governments, to ensure country readiness and effective delivery. The Vaccine Alliance unites developing nations and donor governments, the World Health Organization, UNICEF, the World Bank, the vaccine industry, technical agencies, civil society organizations, the Bill & Melinda Gates Foundation, and other private sector collaborators.
Numerous doctors have expressed concern about the unknown number of Gates-funded NGOs conducting similar trials in India and Africa. They find it troubling how effortlessly these organizations influence our governance and policies, treating us as guinea pigs.
AVOID FUTURE SHOTS!
Also Read: