The agenda of the vaccination campaign will soon be known to the general public. Should governments and the mainstream media still be proud of vaccine-related fatalities? And here we will also reveal the WHO’s complicity in vaccine side effects and disregard for safety concerns.
After several nations reported potential serious side effects, Germany, France, and Italy announced that they would stop using AstraZeneca Covid-19 shots. So why do Indian governments and the media still advocate for vaccines Today?
Our Qvive network has made it its mission to deliver accurate and reliable information to the public.
The Qvive Network has continuously provided accurate information about vaccine adverse reactions with the help of Expert teams and AIM teams and will carry on doing. Truth, Honesty, and Transparency are values that our Qvive Network firmly upholds.
First let us understand What are AEFI CASES?
AEFI cases are of three types. Common and minor AEFI cases include fever, pain and swelling at injection site or irritability. In the next level (severe), such cases do not lead to long-term problems but can be disabling. They include pain and swelling, which spread beyond the nearest joint, or even high-grade fever. Serious AEFI cases are conditions requiring hospitalisation or leading to death or disability. (News18)
Here are a few facts from the past that demonstrate vaccine-related deaths in India.
The Indian Express: Govt panel confirms first death linked to vaccines
The panel, which released data for vaccinations only till the first week of April, examined five deaths reported following vaccination on February 5, eight cases from March 9 and 18 cases from March 31, 2021.
A government panel studying Covid-19 vaccine side effects has confirmed the first death due to anaphylaxis following vaccination. The National AEFI Committee carried out causality assessment of 31 reported AEFI (Adverse Events Following Immunisation) cases, and said the death of a 68-year-old man had been attributed to a severe allergic reaction following vaccination on March 8.
“It is the first death linked to Covid-19 vaccination due to anaphylaxis. It re-emphasises the need to wait for 30 minutes at the inoculation centre after receiving the jab, Dr N K Arora, Chairperson, National AEFI Committee, told PTI.
( https://indianexpress.com/article/india/covid-19-vaccine-death-side-effect-7360141/ )
Times of India: 180 deaths after Covid-19 jabs till March 31, 75% within 3 days.
A presentation made to the National AEFI (adverse event following immunization) Committee on March 3, 2021, recorded that there had been 180 deaths till that time and three-fourths of the deaths happened within three days of the shot.
Many of the AEFIs and deaths reported in India bear striking similarities to those recorded in the European Union and the UK, on the basis of which the European Medicines Agency (EMA ) has warned “healthcare professionals and people receiving the vaccine to remain aware of the possibility of very rare cases of blood clots combined with low levels of blood platelets occurring within 2 weeks of vaccination”.
( https://timesofindia.indiatimes.com/india/180-deaths-after-jabs-till-mar-31-75-within-3-days/articleshow/81978526.cms )
The Hindu: 180 deaths following vaccination reported in India
Individual AEFI cases were presented and brought up for discussion by experts in the AEFI causality assessment committee.
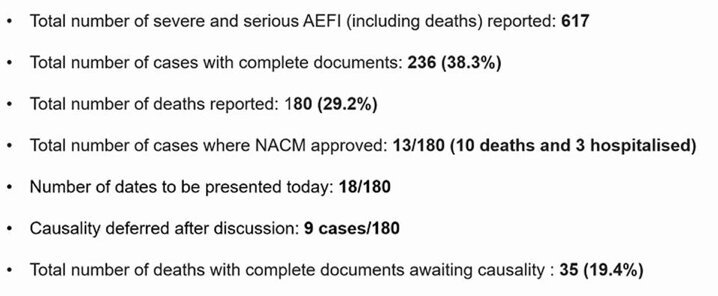
According to a presentation made to the National AEFI Committee during a meeting held on March 31, 2023, there have been 617 severe and serious (including deaths) adverse events following immunization (AEFI). As on March 29, a total of 180 deaths (29.2%) have been reported following vaccination across the country. Complete documentation is available only for 236 (38.3%) cases.
( https://www.thehindu.com/news/national/coronavirus-180-deaths-following-vaccination-reported-in-india/article34274144.ece )
NEWS18:
DEATHS AFTER VACCINATION
Close to 2% (488) of the total AEFI cases (26,200) resulted in deaths, according to data from government sources. A total of 301 men and 178 women were among the dead; the data did not mention the gender of the rest of the nine people.
0.000217: Percentage of deaths in relation to total doses of Covishield (two in every 10 lakh people vaccinated).
• 0.00008: Percentage of deaths in relation to total doses of Covaxin (less than one in every 10 lakh people vaccinated).
• 0.00020: Percentage of deaths in relation to total doses (two in every 10 lakh people vaccinated).
Of the 488 fatalities, 207 were admitted in hospitals. The youngest person reported to have died is a 21-year-old man from Jammu and Kashmir’s Kathua. The oldest is a 97-year-old man from Karnataka’s Kolar, according to the data.
• At least 27 of those who died were less than 39 years old.
• At least 10 of them were below 29.
Among the severe cases, blood in vomit, “sudden unconsciousness”, chest pain and difficulty in breathing were some of the symptoms. There have been a few cases of thrombocytopenia — a condition in which a patient has low blood platelet count. Platelets (thrombocytes) are colorless blood cells that help blood clot.
( https://www.news18.com/news/india/26k-adverse-events-488-deaths-reported-in-india-during-covid-vaccination-drive-data-3845363.html )
Science The Wire: 617 Serious Adverse Events After Vaccination Reported In India Until March 29
Bengaluru: As of March 29, 2021, at least 617 serious adverse events following immunization (AEFI) had been reported from around the country, according to a presentation made before the National AEFI Committee two days later. Of these 617, at least 180 people (29.2%) died, and of these, complete documents were available only for 35 people (19.4%).
According to vaccine scientist Dr. Gagandeep Kang, there are five types of AEFIs: vaccine product-related reaction, vaccine quality defect-related reaction, immunization error-related reaction, immunization anxiety-related reaction, and coincidental event. The National AEFI Committee is tasked with determining the type of each AEFI in the country and, where applicable, arranging for compensation for the affected parties and/or informing vaccine regulation.
The Government of India has been drawing flak for some time after it stopped publishing AEFI reports after February 26, around 40 days after the start of India’s COVID-19 vaccination drive, and after a seemingly laidback response to concerns about AstraZeneca’s shot, called ‘Covishield’ in India.
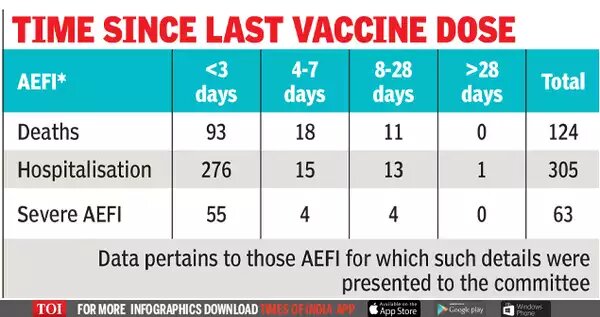
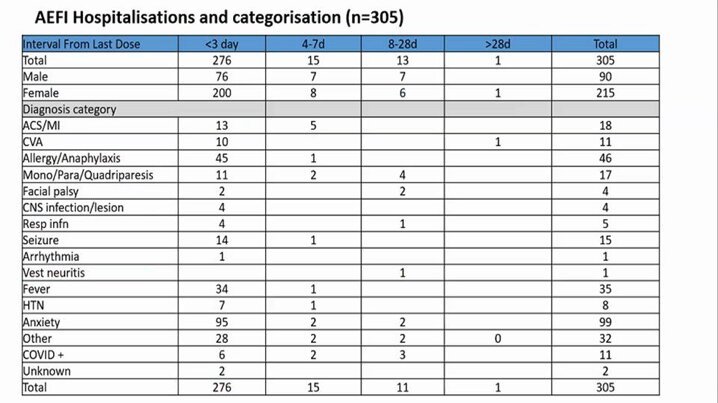
According to the slides presented on March 31, prepared by the Immunisation Technical Support Unit at the health ministry and which The Wire Science has seen, the ministry has ascertained the type of AEFI for 492 reports. Of them, 63 people didn’t require hospitalization, 305 people required hospitalization and 124 people died. A little more than half of those who died did so due to acute coronary syndrome, which refers to any condition that suddenly and significantly reduces blood flow to the heart, including heart attacks.
However, according to the presentation, complete documents were available for only 35 people. These documents refer to case reporting forms and case investigation forms that the corresponding healthcare workers must file at the district level for each case.
“Currently, we are observing gaps in how serious adverse events are being investigated at the district level,” Delhi-based public health researcher Malini Aisola had previously told IndiaSpend on March 9. “In some cases, there is a post-mortem, in some cases, there isn’t.” She told The Hindu on April 9 that “in at least six out of 10 cases where the National AEFI Committee has completed causality assessment, no post mortem has been done”.
On March 17, as The Wire Science reported, “the immunization division of the health ministry released a note (Z.16025/02/2018-IMM) saying it had considered eight AEFIs. A subcommittee had determined four were “coincidental”, one was “unclassifiable” and three were designated B1: “reviewing factors result in conflicting trends of consistency and inconsistency with causal association to immunization”.” All of these cases were among recipients of Covishield.


Dr. Jacob John, formerly of Christian Medical College, Vellore, also pointed to a preliminary pattern in the data – that the incidence of deaths wouldn’t be bunched together in time, and might be more evenly distributed if they were all coincidental. As Prasad Ravindranath, the article’s author, notes, “there are 93 deaths in the first three days and 18 deaths in four-seven days after vaccination. There have been 11 deaths in 8-28 days post-vaccination.” This and similar patterns merit further investigation, according to Dr John.
The presentation doesn’t mention the name of the vaccine for each of the AEFI events, but since last month, there have been widespread concerns in Europe that the AstraZeneca shot may be associated with rare but debilitating blood clots. While authorities in Europe insisted that the shot’s benefits outweighed its risks and that people should continue receiving it, some governments as well as an assessment body of the European Medicines Agency (EMA) said there could be a very small risk factor for cerebral venous sinus thrombosis.
According to The Hindu, the EMA “included only six deaths from India after vaccination with Covishield for its analysis” because, Aisola said, of “a massive backlog in processing assessments in India”. In addition, Dr. Kang also said in an interview with Karan Thapar for The Wire last week that while the risk is low, the issue has been compounded by the Indian government’s secretive deliberations on the matter.
( https://science.thewire.in/health/617-serious-adverse-events-after-vaccination-reported-in-india-until-march-29/ )
Also Read:
