There are many ethical concerns surrounding the development of a COVID-19 vaccine. How can we ensure that testing is done ethically on humans? Is it acceptable to mandate the vaccine for those who are hesitant?
The use of fetal tissue from aborted human beings in medical research predicates the health of some on the deliberate destruction of the lives and health of others. That predication is incompatible with the fundamental commitments of medicine. In the face of this global crisis, we must hold to our ethical principles more firmly than ever.
Research groups around the world are working to developed more than 130 candidate vaccines against COVID-19, according to the World Health Organization. At least six of those candidates use one of two human fetal cell lines: HEK-293, a kidney cell line widely used in research and industry that comes from a fetus aborted in about 1972; and PER. C6, a proprietary cell line owned by Janssen, a subsidiary of Johnson & Johnson, developed from retinal cells from an 18-week-old fetus aborted in 1985. Both cell lines were developed in the lab of molecular biologist Alex van der Eb at Leiden University.
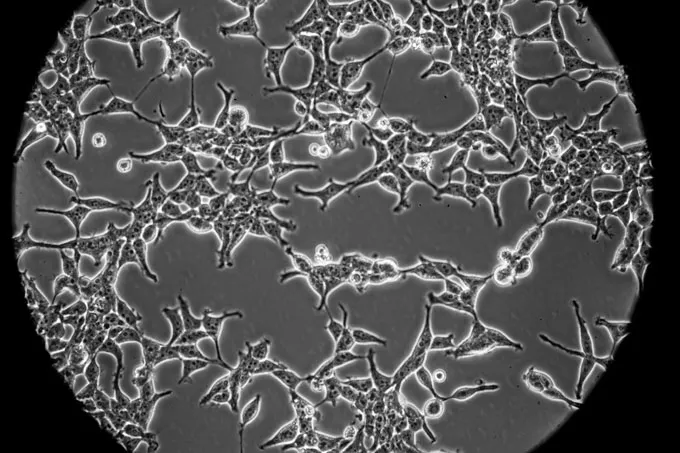
Two of the six vaccines have entered human trials (see table, below). Five are made by using human fetal cells as “factories” to make adenoviruses that carry genes from SARS-CoV-2, the virus that causes COVID-19. The adenoviruses, which are disabled so they can’t replicate, are given as a vaccine; recipients’ cells then produce proteins from the coronavirus, hopefully triggering a protective immune response.
The sixth vaccine, had entered human trials, is a protein subunit vaccine. Researchers use HEK-293 cells to make pieces of the spike protein that studs the coronavirus’ surface. To trigger an immune response, the vaccine is delivered through a skin patch with 400 tiny needles.

Researchers have been studying the expression of hybrid proteins in mammalian cell lines. By utilizing advanced techniques and cutting-edge technology, they are able to get insights into how these proteins function and interact within the cell.
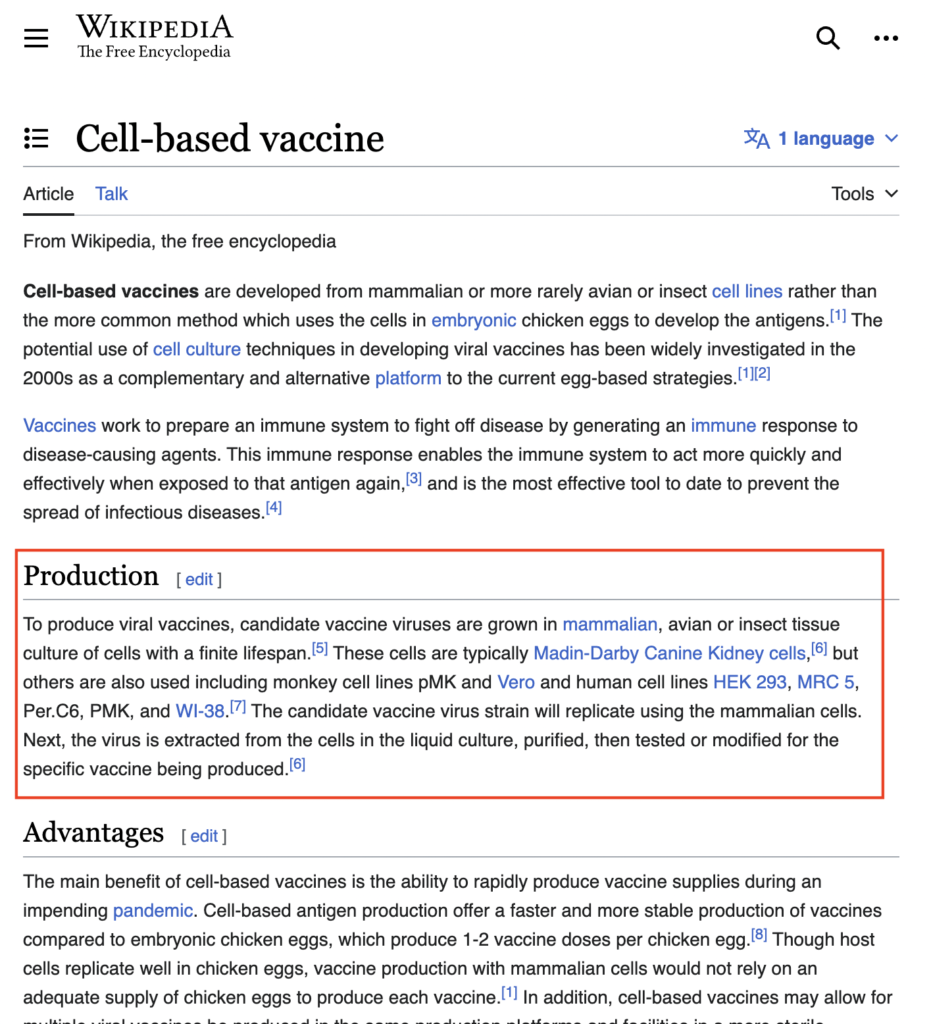
When conducting studies on hybrid protein expression, researchers have the option to use a variety of mammalian cell lines, each with its own unique characteristics. Some of the most commonly used mammalian cell lines include:
- HeLa Cells: Derived from a cervical cancer patient in the 1950s, HeLa cells are widely utilized in research due to their rapid growth and high transfection efficiency.
- CHO Cells: Chinese Hamster Ovary (CHO) cells are commonly used for the production of recombinant proteins due to their ability to perform complex post-translational modifications.
- HEK293 Cells: Human Embryonic Kidney (HEK) 293 cells are commonly utilized for the expression of membrane proteins and are ideal for studying protein-protein interactions.
- Vero Cells : derived from African green monkey kidney cells, are commonly used in vaccine production due to their ability to grow rapidly and support the replication of a wide range of viruses.
Are Fetal Cells Used to Make Vaccines?

Several brands are involved in utilizing mammalian cell lines for COVID-19 vaccine research. One such brand is Moderna, which has developed a COVID-19 vaccine based on mRNA technology. By leveraging mammalian cell lines, Moderna has been able to produce the necessary viral proteins for their vaccine effectively and efficiently.
Pfizer, which has collaborated with BioNTech to develop a COVID-19 vaccine in clinical trials. The use of mammalian cell lines has been instrumental in the production of this vaccine, allowing for rapid scaling up of manufacturing processes.
IISc-incubated Mynvax to get funding to bring “warm” Covid vaccine to market

Researchers at the Indian Institute of Science in Bengaluru have developed a new type of COVID-19 vaccine that does not require cold storage. Called a “warm vaccine”, it is designed using a hybrid protein called RS2 that is made up of two parts of the SARS-CoV-2 spike protein. In animal tests, this vaccine was found to trigger a strong immune response and provide better protection than vaccines using the full spike protein. The RS2 antigen can also be stored at room temperature for a month without refrigeration. This makes the distribution and storage of the vaccine much more economical than others currently available. If successful in human trials, this warm vaccine has the potential to offer protection against existing COVID variants as well as any future variants that may emerge.
The researchers used mammalian cell lines to study the expression of the hybrid protein.
“The protein showed very high levels of expression, and I (initially) thought that the experiment was not working properly,” said Nidhi Mittal, PhD student at MBU and first author of the study.
The team then tested the effects of the protein in both mice and hamster models. They found that the hybrid protein triggered a strong immune response and provided better protection when compared to vaccines containing the whole spike protein.

The CEO of Mynvax Lab is Dr. Raghavan Varadarajan, said that his team began working on the vaccine even before the pandemic became widespread in India. “At that time, the Bill and Melinda Gates Foundation provided funding and support,” he said,
Since 2000, Varadarajan’s team has been working on designing several viral vaccines, including those against AIDS and influenza. They have leveraged this expertise to design their current RS2-based COVID-19 vaccine candidate in collaboration with the startup Mynvax, which was, until recently, incubated at IISc.

Founded in 2017 by Dr Raghavan Varadarajan, Professor at IISc, Bangalore, and Dr Gautham Nadig, an alumnus of the institute, Mynvax aims to develop novel recombinant vaccines to fight the human influenza virus.
“In addition to expeditiously advancing its existing vaccine candidates, both in India and overseas, the company would also invest in developing new vaccine modalities,” Dr Nadig, Co-founder and Executive Director, Mynvax, said in a statement. He also revealed that Mynvax is looking to build partnerships with large vaccine manufacturers to accelerate the deployment of the vaccines.
“With the support of BIRAC, Government of India, early-stage investors, and SID, IISc, it was able to rapidly demonstrate its ability to develop novel vaccine candidates, which have been rigorously tested for performance and unique attributes such as heat tolerance that enhance access to those in need,” the startup said in a statement.
Mynvax Private Limited is a biotechnology company in the pre-clinical stage of vaccine development. They have a strong focus on creating a recombinant vaccine for human influenza and COVID-19. Through genetic engineering, Mynvax has developed a vaccine targeting the receptor-binding domain of the SARS-CoV-2 virus. This domain attaches to the Ace2 receptor found on the surface of target cells in the human respiratory tract.

Where is Mynvax’s headquarters?
Mynvax is based out of Bengaluru.
When was Mynvax founded?
Mynvax was founded in the year 2017.
What is the legal name of Mynvax?
The legal name of Mynvax is Mynvax Private Limited.
Who are the founders of Mynvax?
Mynvax is founded by Gautham Nadig and Raghavan Varadarajan.
Which VC Investors & Angel Networks funded Mynvax?
Mynvax is funded by VC Investors & Angel Networks like 1crowd and 3 others.
Which Incubators & Accelerators supported Mynvax?
Mynvax is supported by Society For Innovation And Development, Iisc Bangalore.
What are the focus areas of Mynvax?
The key focus areas of Mynvax are COVID-19 vaccine and 3 others.
Who are Mynvax’s competitors?
Possible competitors of Mynvax may include Biocor, Getsetplay, Kyntox Biotech India Pvt Ltd, Kotak Investment Advisors, and 7 others.
It’s Unethical to Use Fetal Tissue in COVID-19 Research
In June 2019, the Department of Health and Human Services announced that the federal government would adopt a new approach to research that used fetal tissue obtained from elective abortions. Intramural research projects using such tissue—i.e., those conducted by the National Institutes of Health—would be discontinued. Funded extramural projects already underway would be allowed to continue through their grant periods, but new extramural projects would need to be considered by an ethics advisory panel to determine “whether, in light of the ethical considerations, NIH should fund the research project.” HHS identified the Trump administration’s core concern: “Promoting the dignity of human life from conception to natural death is one of the very top priorities of President Trump’s administration.”
That ethics advisory panel has yet to be convened. For now, no new extramural research is being funded by the NIH that involves fetal tissue from elective abortions. This is an important success for the pro-life cause.
In at least one prominent case, however, the new policy has restricted active research on a project of relevance to our current COVID-19 health crisis: researcher Kim Hasenkrug has sought permission [a] to pursue research on “humanized mice,” mice whose lungs have been made “human-like” using tissue obtained from aborted human fetuses. This research could be helpful in generating therapies for COVID-19 patients.
[a] https://www.vox.com/policy-and-politics/2020/3/19/21186743/coronavirus-treatment-research-delay-trump-fetal-tissue-banScientist Calls Use of Fetal Tissue in Medical Research Essential
Source: Thehindu, Linkedin, Public Discourse, Yourstory, IISC,Youtube,Wikipedia,
Also Read:
