Bill Gates has often mentioned the threat of a smallpox attack. Where did he get that idea? Was this the first event before Event 201, where leaders from Big Tech, Big Pharma, Fauci, Gates, Bloomberg, and others gathered to prepare? Or was it just a prediction he made years before 2019 about a pandemic? It seems like everything has been set up in advance. The origins of this situation didn’t start in China but in government labs funded by taxpayers, like UNC and even the CDC in Atlanta. This isn’t just a conspiracy theory; it’s the reality.

Some official papers say that Monkeypox might be hiding the problems caused by COVID vaccines, which could lead to issues like Shingles, Autoimmune Blistering Disease, and Herpes Infection.

Isn’t it strange that for 50 years, monkeypox didn’t spread much beyond a few African countries? But then, just two years after Covid-19 appeared, monkeypox suddenly shows up in every Western countries and is being talked about a lot by health officials and the media?
The Director General of the World Health Organization, Tedros Adhanom Ghebreyesus, has recently made a big decision by declaring monkeypox a Public Health Emergency of International Concern, going against the usual procedures of the organization.

One of the side effects linked to the COVID-19 vaccines is the reactivation of the varicella-zoster virus. This can show up as herpes, small pox, chickenpox, autoimmune blisters, or even monkeypox. Interestingly, monkeypox can look a lot like smallpox / chickenpox, and when it appears in adults, it’s known as shingles.
Few documents show that COVID jabs reactivate varicella-zoster virus, resulting in what they are now calling monkeypox
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) websites explain that monkeypox is similar to smallpox.
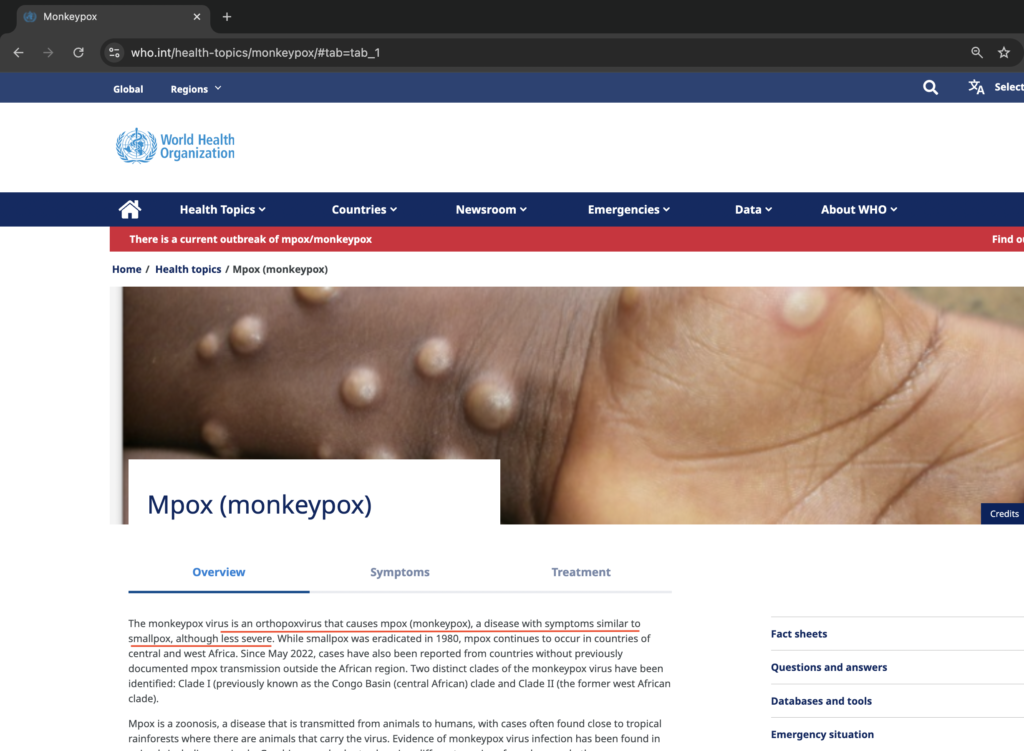
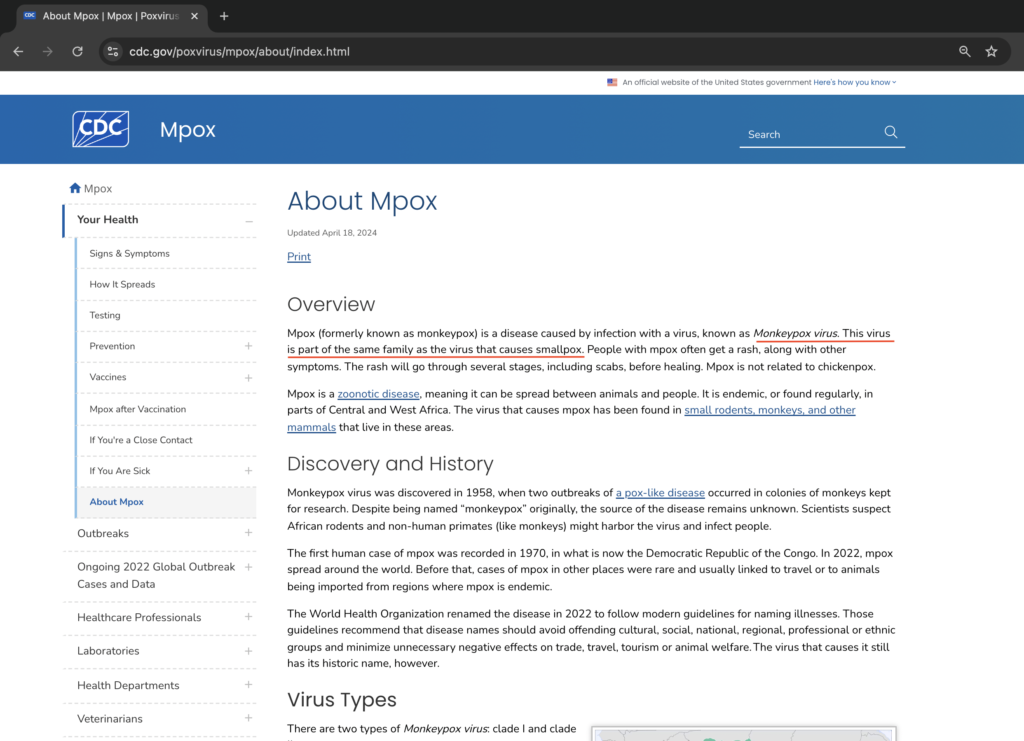
A study from 1988 pointed out that telling monkeypox apart from smallpox / chickenpox can be tricky. So, what some people are calling monkeypox might actually be smallpox or chickenpox or shingles, depending on how old they are. This could be related to the COVID vaccines that were rolled out quickly before the recent monkeypox cases. The media and government say that monkeypox, sometimes called pridepox, is mostly spreading in the LGBT community [a]. However, it’s possible that the real source could be the COVID shots, which many in the LGBT community received . Exposé News [b] explains that the chickenpox virus, known as varicella-zoster, stays in the body for life, similar to the herpes simplex virus. It can remain hidden in nerve cells for years and then reactivate, causing painful shingles, which is a blistering rash. Unfortunately, or maybe fortunately, depending on whether you got the COVID-19 vaccine, official government data and secret Pfizer documents suggest that the vaccine might be waking up the dormant smallpox /chickenpox or herpes virus because of the serious harm it can do to the immune system.
[a] https://naturalnews.com/2022-07-21-uk-targeting-children-monkeypox-vaccines-gay-behavior.html [b] https://expose-news.com/2022/07/26/monkeypox-coverup-covid-vaccine-immune-system-damage/July 27, 2022: Data from past studies show that the smallpox vaccine is about 85% effective in preventing monkeypox, the WHO and CDC say
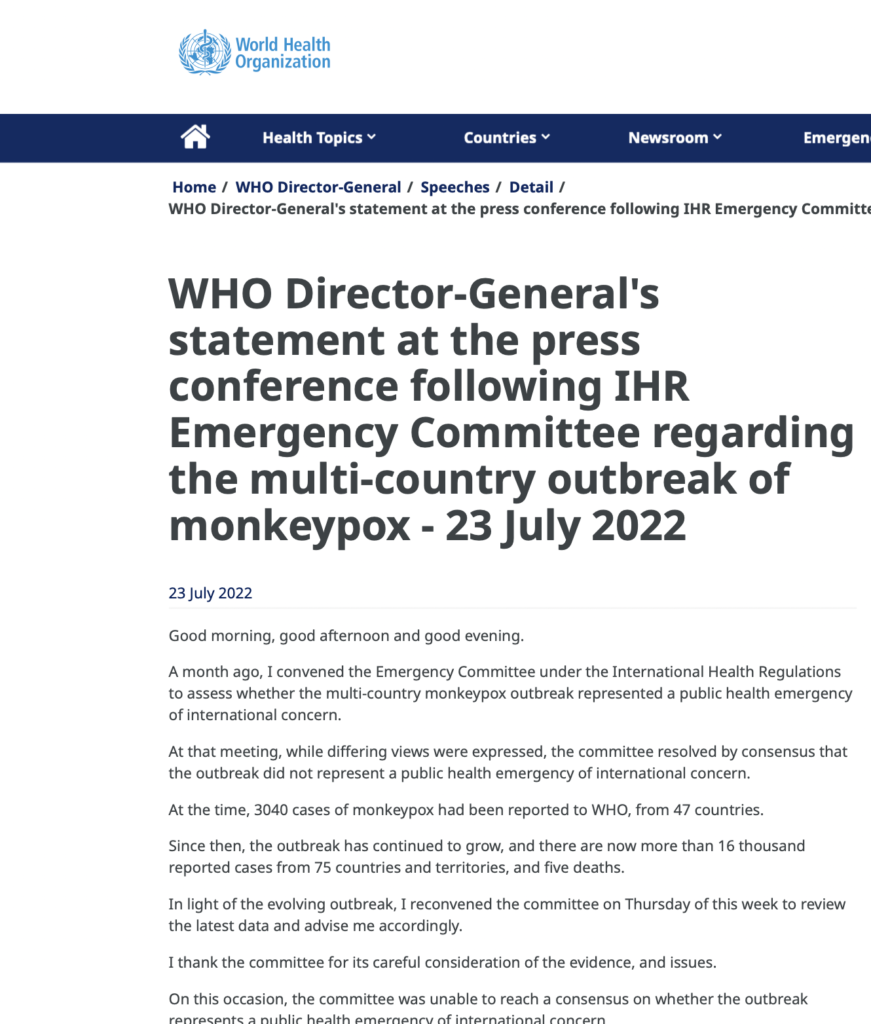
The World Health Organization (WHO) recently declared the multi-country monkeypox outbreak a global public health emergency.
This comes as tens of thousands of cases have been reported in more than 70 countries, with several thousand of those cases in the U.S.
As cases have increased worldwide, some people are searching for ways to prevent infection. Claims on social media say that smallpox vaccines offer protection against monkeypox infection.
Since monkeypox is “closely related” to the virus that causes smallpox, smallpox vaccines “can protect people from getting monkeypox,” according to the Centers for Disease Control and Prevention (CDC).
Data from past studies show that the smallpox vaccine is about 85% effective in preventing monkeypox when given before someone is exposed to the virus, the World Health Organization (WHO) and CDC say. The vaccine can also be effective at preventing illness or reducing symptom severity if it is given shortly after someone is exposed to monkeypox.
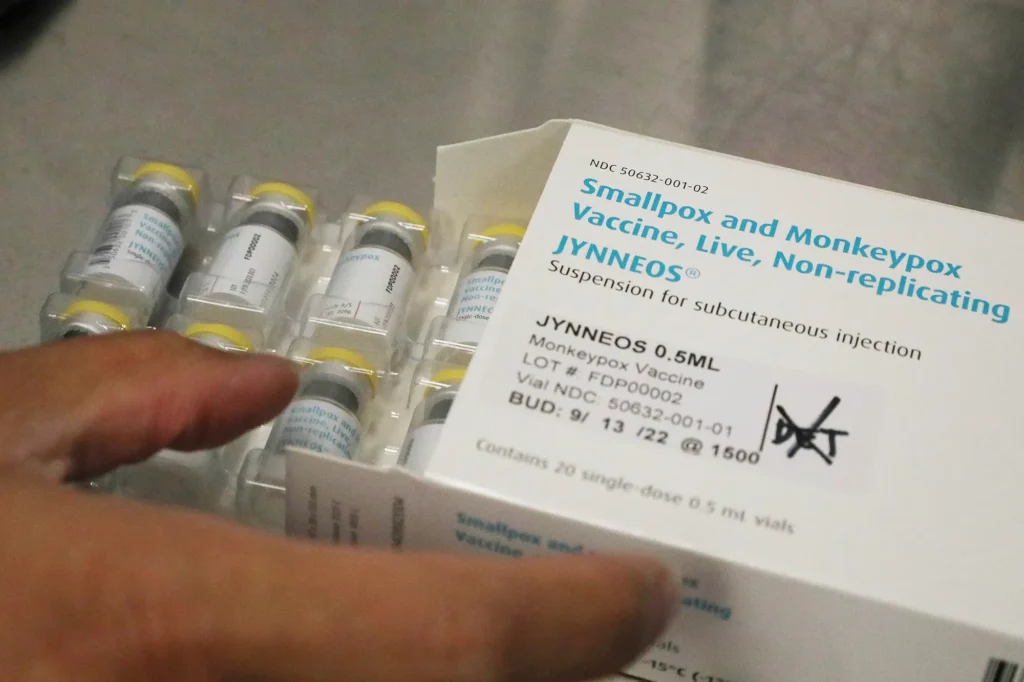
The U.S. Food and Drug Administration (FDA) has licensed two vaccines to prevent smallpox: ACAM2000 and JYNNEOS.
JYNNEOS
- Also known as Imvamune or Imvanex in other countries.
- Approved for the prevention of smallpox and mpox.
- The FDA approved JYNNEOS in 2019 for use in people ages 18 years and older and determined to be at high risk for smallpox or mpox infection.
Ref: https://www.cdc.gov/smallpox/vaccine-basics/vaccination-effects.html
The ACAM2000 vaccine is approved by the FDA for use in smallpox prevention, but the CDC has expanded access to allow its use for monkeypox as well, according to the U.S. Department of Health and Human Services.
The JYNNEOS vaccine was approved by the FDA in September 2019 for use in the prevention of both smallpox and monkeypox disease. People with inflammatory skin conditions such as eczema, as well as those with weakened immune systems, should only use the JYNNEOS vaccine, the CDC says.
President Joe Biden’s administration has ramped up distribution of both vaccines, but they aren’t readily available to everyone. The vaccines are limited right now to those who are most at risk of being infected with monkeypox, including people with who were exposed to someone who has monkeypox, people who have had multiple or anonymous sexual partners, and men who have sex with men.
The Biden-Harris Administration announced the first phase of its national monkeypox vaccine strategy:

Routine smallpox vaccinations used to be commonplace in the United States, but this practice stopped in 1972 after the disease was eradicated in the country, the CDC says. That means smallpox vaccines aren’t available to the general public anymore. Those who have previously been vaccinated against smallpox can usually find evidence as a scar on their upper arm, according to the WHO.
So what does a previous smallpox vaccination mean during the current monkeypox outbreak?
Though previous vaccination provides protection against monkeypox, it may not be lifelong, according to the CDC. During the 2003 monkeypox outbreak and the current outbreak, some people who had received the smallpox vaccine decades ago were infected with monkeypox.
Stuart Ray, M.D., a professor at Johns Hopkins Medicine, agrees.
“What we don’t know is whether or at what point people who have had decades since that [smallpox] vaccination, or have become immunosuppressed since that vaccination, might become susceptible,” he said.
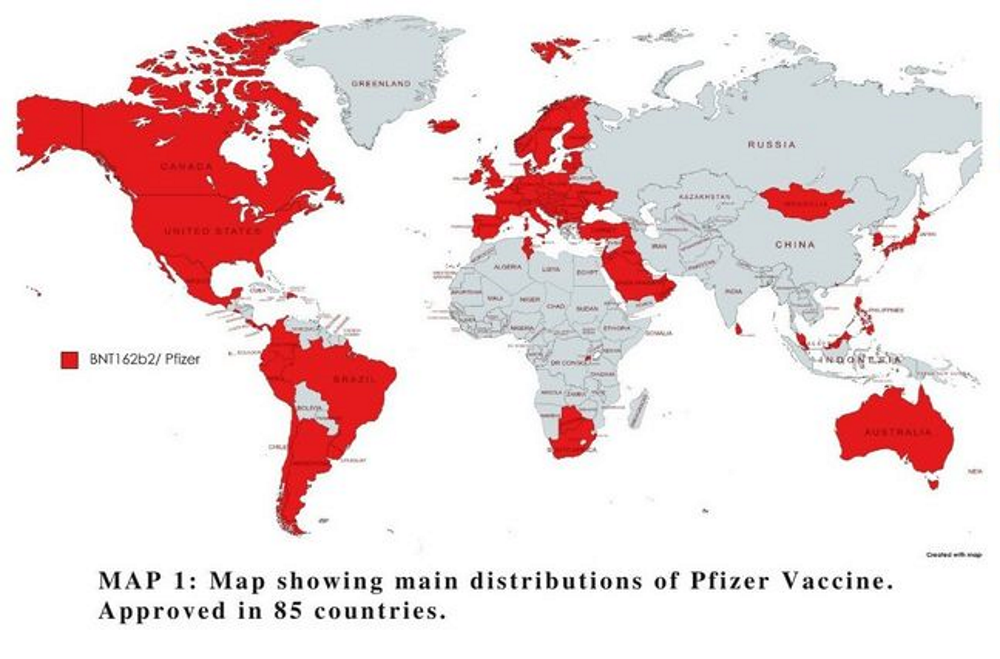
A map showing the main distributions of the Pfizer vaccine.
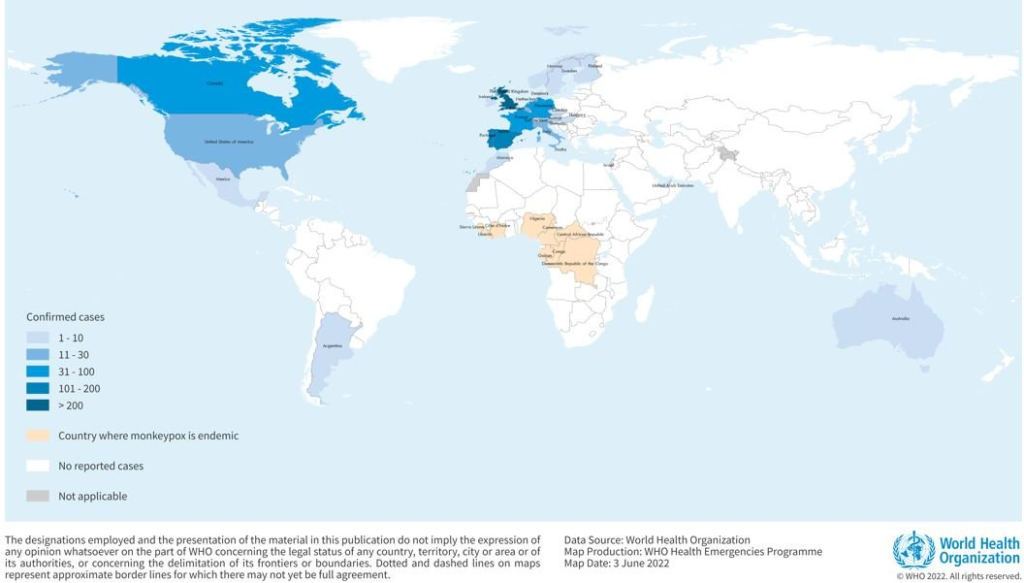
Many countries where “confirmed” cases of monkeypox have been reported to the World Health Organization (WHO) since the middle of May 2022 –
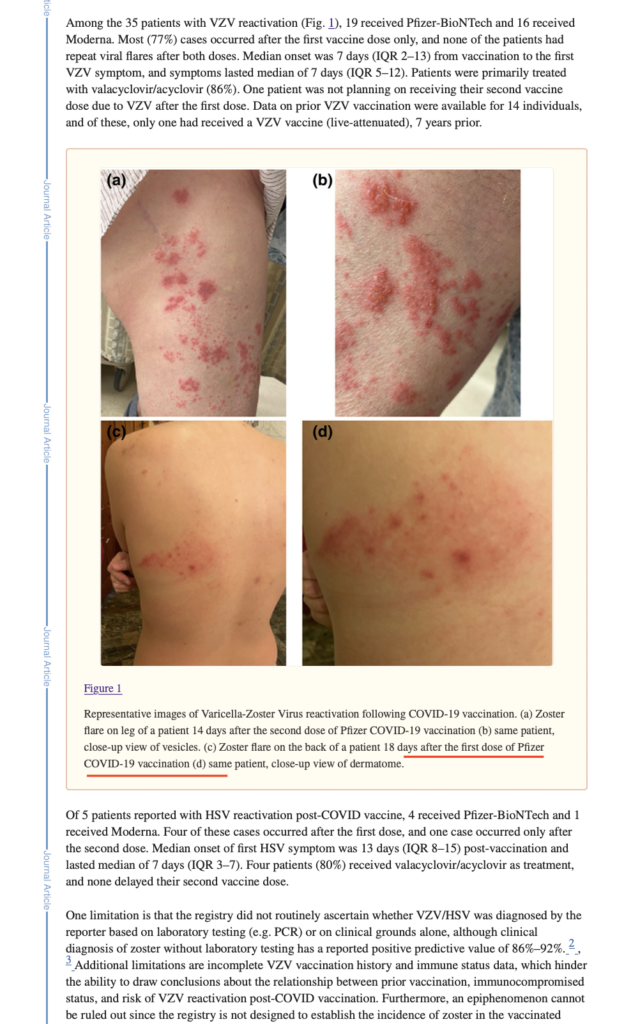
Few documents show that COVID jabs reactivate varicella-zoster virus, resulting in what they are now calling monkeypox
- Representative images of Varicella‐Zoster Virus reactivation following COVID‐19 vaccination:

Planned Strategy:
Sweden competed at the 2024 Summer Olympics in Paris from 26 July to 11 August 2024.
On 14 August 2024, the World Health Organization declared Mpox a global public health emergency for the second time in two years. The declaration comes after the sudden surge of cases from the Democratic Republic of Congo (DRC) that has now spread to other countries.
In the latest development, Sweden reported its first mpox case, becoming the first country outside the African continent to report the more contagious strain of mpox virus. More than 15,600 cases and 537 deaths have been reported so far this year alone in Africa.
Should this hypothesis hold true, it suggests that the Western countries are experiencing a surge in shingles cases induced by the COVID-19 vaccine, alongside other symptoms linked to the reactivation of the varicella-zoster virus. This phenomenon raises significant concerns regarding vaccine safety and its potential side effects.
Reports indicate that the U.S. Food and Drug Administration (FDA) was aware of these implications, which may explain its decision to withhold Pfizer’s COVID-19 vaccine safety and trial documentation for a period of 75 years. However, judicial intervention has mandated the earlier release of these documents, shedding light on the situation.
Within the released documents, a section detailing adverse events of “special interest” (AESI) identifies herpes viral infections as a possible outcome of the COVID-19 vaccine, alongside inflammation. Notably, by February 2021, just two months post the emergency use authorization of the Pfizer vaccine in the U.S. and the U.K., there were 8,152 reported cases of herpes infections, with 18 instances resulting in multiple organ dysfunction syndrome (MODS).
Multiple Organ Dysfunction Syndrome is characterized by a systemic inflammatory response that necessitates prolonged intensive care unit (ICU) treatment and is associated with a high mortality rate, contingent upon the number of organs affected. Herpes infections have been identified as a potential cause of this serious condition.
Additional data from the Centers for Disease Control and Prevention (CDC) indicate a significant increase in cases of herpes, shingles, and multiple organ dysfunction syndrome in the United States following the implementation of Operation Warp Speed. The immunosuppressive effects of the COVID-19 vaccines may act as a catalyst for the reactivation of dormant herpes viruses and varicella-zoster, leading to a resurgence of these diseases.
CDC: Varicella vaccine side effects (chickenpox)
What are the risks from chickenpox vaccine?
- Sore arm from the injection, redness or rash where the shot is given, or fever can happen after varicella vaccination.
- More serious reactions happen very rarely. These can include pneumonia, infection of the brain and/or spinal cord covering, or seizures that are often associated with fever.
- In people with serious immune system problems, this vaccine may cause an infection that may be life-threatening. People with serious immune system problems should not get varicella vaccine.
It is possible for a vaccinated person to develop a rash. If this happens, the varicella vaccine virus could be spread to an unprotected person. Anyone who gets a rash should stay away from infants and people with a weakened immune system until the rash goes away. Talk with your health care provider to learn more.
Some people who are vaccinated against chickenpox get shingles (herpes zoster) years later.
People sometimes faint after medical procedures, including vaccination. Tell your provider if you feel dizzy or have vision changes or ringing in the ears.
As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death.
Ref: https://www.cdc.gov/vaccines/hcp/current-vis/varicella.html
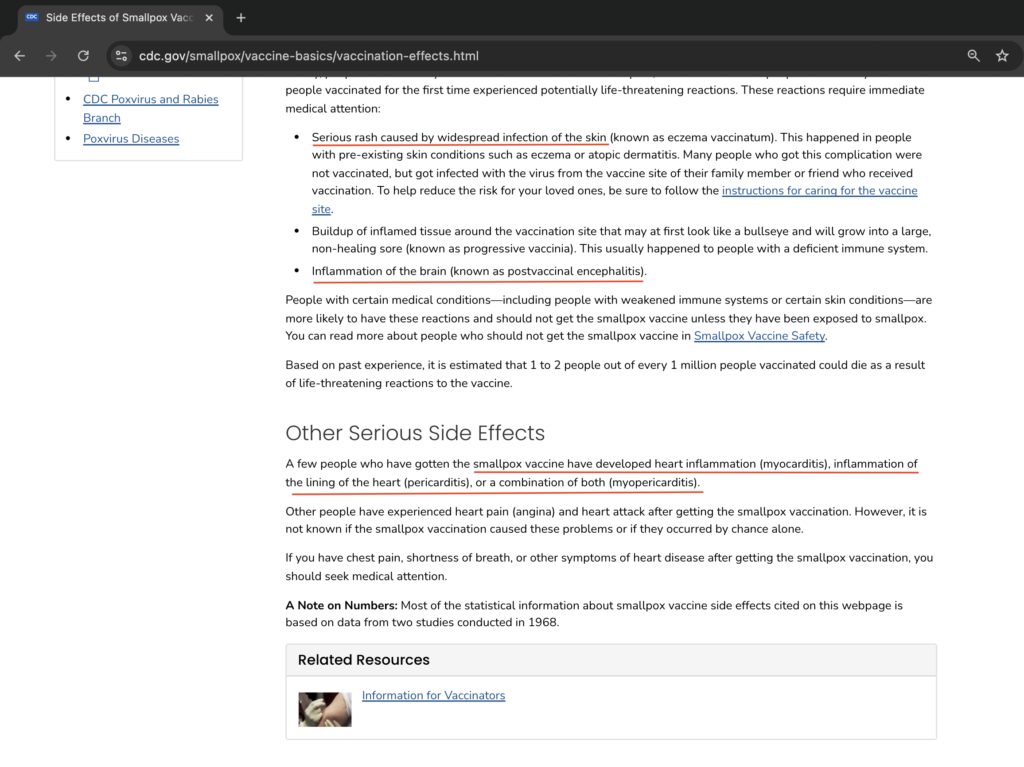
CDC Report: Side Effects of Smallpox Vaccination
Related Article:
#herpesawarenessday
— Herpes Advocate (@HerpesAdvocate) October 13, 2021
HSV-2 is a leading cause of viral meningitis and the most commonly recognized infectious cause of benign, recurrent meningitis. Aseptic meningitis occurs in 36% of women with primary HSV-2 genital infection and 13% of men. pic.twitter.com/oMAKObiDCL
Smallpox/Monkeypox Vaccine (JYNNEOSTM): What You Need to Know
Download: https://www.cdc.gov/vaccines/hcp/current-vis/downloads/smallpox-monkeypox.pdf
Additional Information:
Drugs: Varicella virus vaccine Side Effects
Serious side effects
Along with its needed effects, varicella virus vaccine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur while taking varicella virus vaccine:
More common
- Fever over 39°C (102°F)
Less common
- Blue lips and fingernails
- chest pain or tightness
- chickenpox-like skin rash
- coughing that sometimes produces a pink frothy sputum
- decreased urine output
- difficult, fast, or noisy breathing
- dilated neck veins
- extreme tiredness or weakness
- general feeling of discomfort or illness
- increased sweating
- irregular breathing
- irregular heartbeat
- irritability
- pale skin
- swelling of the ankles, face, fingers, feet, or lower legs
- weight gain
- Black, tarry stools
- blood in the urine or stools
- chills
- confusion
- cough
- difficulty with breathing or swallowing
- fever
- hives
- itching, especially of the feet or hands
- muscle or joint pain
- pinpoint red spots on the skin
- reddening of the skin, especially around the ears
- seizures with high fever
- severe or continuing headache
- stiff neck
- swelling of the glands in the neck
- thickening of bronchial secretions
- unusual bleeding or bruising
- unusual tiredness or weakness, sudden and severe
- vomiting
Incidence not known
- Back pain, sudden and severe
- bleeding gums
- blistering, peeling, or loosening of the skin
- bloating or swelling of the face, arms, hands, lower legs, or feet
- bloody nose
- blurred vision
- bruising more easily
- dizziness
- fast heartbeat
- headache
- heavier menstrual periods
- inability to move the arms and legs
- inability to speak
- large, flat, blue, or purplish patches in the skin
- large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, or sex organs
- loss of bladder control
- muscle spasm or jerking of all extremities
- painful blisters on the trunk of the body
- painful knees and ankles
- pale skin
- pinpoint red spots on the skin
- puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue
- raised red swellings on the skin, buttocks, legs, or ankles
- red, irritated eyes
- red skin lesions, often with a purple center
- seizures
- shakiness and unsteady walk
- skin rash
- slurred speech
- sores, ulcers, or white spots in the mouth or on the lips
- stomach pain
- sudden loss of consciousness
- sudden numbness and weakness in the arms and legs
- swollen or painful glands
- temporary blindness
- tingling of the hands or feet
- unsteadiness, trembling, or other problems with muscle control or coordination
- unusual weight gain or loss
- weakness in the arm or leg on one side of the body, sudden and severe
- weakness of the muscles in your face
Other side effects
Some side effects of varicella virus vaccine may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects.
Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
More common
- Fever of 37.7°C (100°F) or higher, but not above 39°C (102°F)
- hives, itching, pain, redness, soreness, tenderness, or warmth at the injection site
- Common cold
- congestion
- constipation
- cracked, dry, or scaly skin
- diaper rash
- diarrhea
- disturbed sleep
- dry skin
- earache
- heat rash or prickly heat
- lack or loss of strength
- loss of appetite
- muscle ache, cramp, or stiffness
- nausea
- nervousness
- runny nose
- skin rash, encrusted, scaly, and oozing
- sneezing
- sore throat
- stuffy nose
- swelling
- swollen joints
- teething
Incidence not known
- Bacterial skin infections
- body aches or pain
- burning, crawling, itching, numbness, prickling, “pins and needles”, or tingling feelings
- difficulty with moving
- dryness or soreness of the throat
- hoarseness
- pain, redness, swelling, tenderness, or warmth on the skin
- red rash with watery, yellow-colored, or pus-filled blisters
- thick yellow to honey-colored crusts
- voice changes
General
The most common adverse events were injection site reactions and fever.
Very common (10% or more): Fever (27%), otitis media (12%)
Uncommon (0.1% to 1%): Otitis, viral infection, asthenia, hematoma, malaise
Rare (less than 0.1%): Ear pain, infection, candidiasis, non-venomous bite/sting, heaviness
Frequency not reported: Insect bites
Local
Very common (10% or more): Injection site complaints (pain/soreness, swelling and/or erythema, rash, pruritus, hematoma, induration, stiffness) (32.5%)
Common (1% to 10%): Varicella-like rash (injection site)
Uncommon (0.1% to 1%): Injection site ecchymosis, induration
Rare (less than 0.1%): Extravasation, injection site eczema, lump, warmth, stiffness, pain/tenderness/soreness, warm sensation, warm to touch, venipuncture site hemorrhage
Respiratory
Very common (10% or more): Upper respiratory infection (26.9%), cough (11%)
Common (1% to 10%): Rhinorrhea
Uncommon (0.1% to 1%): Nasal congestion, respiratory congestion, influenza, pharyngitis
Rare (less than 0.1%): Pneumonitis, sinusitis, sneezing, pulmonary congestion, epistaxis, rhinitis, wheezing, bronchitis, respiratory infection, pneumonia, flu-like illness
Frequency not reported: Upper respiratory illness, lower respiratory illness
Dermatologic
Common (1% to 10%): Varicella-like rash (generalized), rash, measles/rubella-like rash
Uncommon (0.1% to 1%): Varicella, viral exanthema, contact dermatitis, diaper rash, erythema, miliaria rubra, pruritus, urticaria
Rare (less than 0.1%): Flushing, vesicle, atopic dermatitis, eczema, acne, herpes simplex, contusion, dermatitis, drug eruption, impetigo, skin infection, measles, sunburn, lump, warmth, discoloration, inflammation, roughness/dryness, hive-like rash, hyperpigmentation
Postmarketing reports: Varicella (vaccine strain), Stevens-Johnson syndrome, erythema multiforme, Henoch-Schonlein purpura, secondary bacterial infections of skin and soft tissue including cellulitis, herpes zoster
Psychiatric
Common (1% to 10%): Irritability
Uncommon (0.1% to 1%): Crying, insomnia, sleep disorder
Rare (less than 0.1%): Apathy, nervousness, agitation, dream abnormality, emotional changes
Frequency not reported: Disturbed sleep
Gastrointestinal
Uncommon (0.1% to 1%): Gastroenteritis, diarrhea, vomiting
Rare (less than 0.1%): Abdominal pain, nausea, flatulence, hematochezia, mouth ulcer, lip abnormality
Frequency not reported: Constipation, cold/canker sore
Nervous system
Uncommon (0.1% to 1%): Headache, somnolence, fatigue
Rare (less than 0.1%): Febrile seizures, hypersomnia, gait abnormality, tremor
Postmarketing reports: Encephalitis, cerebrovascular accident, transverse myelitis, Guillain-Barré syndrome, Bell’s palsy, ataxia, non-febrile seizures, aseptic meningitis, dizziness, paresthesia
Hematologic
Rare (less than 0.1%): Lymphadenopathy, lymphadenitis, thrombocytopenia
Postmarketing reports: Aplastic anemia, idiopathic thrombocytopenic purpura
Hypersensitivity
Frequency not reported: Allergic reactions (including allergic rash, hives)
Postmarketing reports: Anaphylaxis (including anaphylactic shock) and related phenomena such as angioneurotic edema, facial edema, and peripheral edema; anaphylaxis in individuals with or without an allergic history[
Ocular
Uncommon (0.1% to 1%): Conjunctivitis
Rare (less than 0.1%): Acute conjunctivitis, tearing, edema of the eyelid, irritation
Frequency not reported: Eye complaints
Postmarketing reports: Necrotizing retinitis (in immunocompromised individuals)
Metabolic
Uncommon (0.1% to 1%): Anorexia[
Uncommon (0.1% to 1%): Anorexia
Rare (less than 0.1%): Musculoskeletal pain, myalgia, stiffness, pain of the hip, leg, or neck
Frequency not reported: Chills, stiff neck, arthralgia
Ref: https://www.drugs.com/sfx/varicella-virus-vaccine-side-effects.html#refs
Source: Wikipedia
Also Read:
