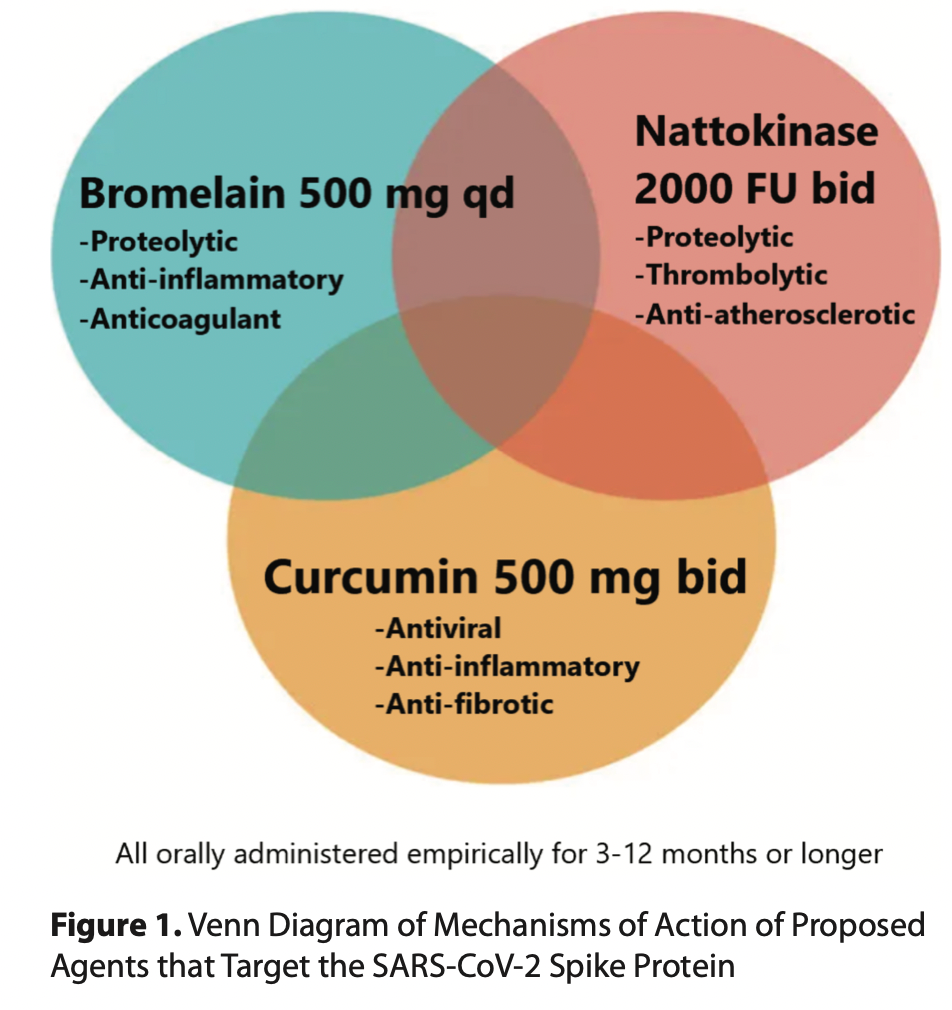
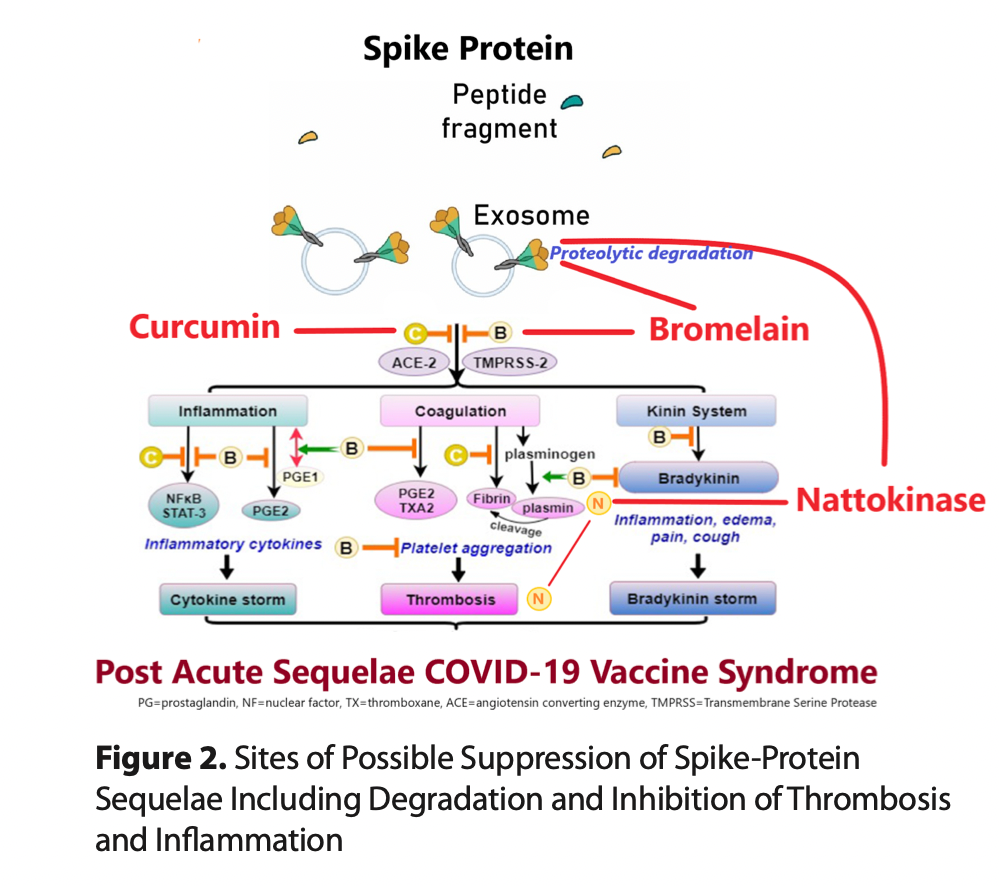
Publication of Baseline Protocol for Those Suffering from Long-COVID and Post-Acute Sequelae after COVID-19 Vaccination
For several weeks I have been messaging the scientific community and the public about an approach addressing the burden of SARS-CoV-2 Spike protein in tissues and organs in the human body that is largely responsible for post-COVID and vaccine injury syndromes.
No therapeutic claims can be made since large, prospective, double-blind randomized, placebo-controlled trials have not been completed on any of the compounds mentioned in this paper. I checked clinicaltrials.gov and no such trials have been planned. The Biden HHS US Action Plan ( https://open.substack.com/pub/petermcculloughmd/p/us-hhs-national-action-plan-on-long?r=14jb45&utm_campaign=post&utm_medium=web ) for Long-COVID Research has pumped a billion dollars into long-COVID research and no new therapies have emerged. HHS, NIH, CDC, FDA have not recognized the larger issue of vaccine damage to the body.
At three and one half years into the pandemic and two and a half years into the COVID-19 vaccine debacle, myself and my clinic partners formulated a baseline regimen upon which additional drugs or agents can be added. We believe the Spike protein and the inflammation cause by it and its proteolytic fragments are at the heart of the pathophysiology we are observing.
Clinical Rationale for SARS-CoV-2 Base Spike Protein Detoxification in Post-COVID-19 and Vaccine Injury Syndromes
The spike protein is responsible for the pathogenicity of the SARS-CoV-2 infection and drives the development of adverse events, injuries, disabilities, and death after vaccination through immunologic and thrombotic mechanisms.
CLICK HERE The long-lasting spike protein has been found in the brain, heart, liver, kidneys, ovaries, testicles, and other vital organs at autopsy in cases of death after vaccination. In the case of vaccine-induced thrombotic injury, the spike protein has been found within the blood clot itself. Thus, there is a strong rationale for considering residual SARS-CoV-2 spike protein as a treatment target in post-COVID-19 and vaccine injury syndromes. The spike protein participates directly in pathophysiology, incites inflammation, and propels thrombosis. While specific syndromes (cardiovascular, neurological, endocrine, thrombotic, immunological) will require additional therapies, we propose the clinical rationale for a base detoxification regimen of oral nattokinase, bromelain, and curcumin for patients with post-acute sequelae from SARS-CoV-2 infection and COVID-19 vaccination. We searched the literature for all available sources of evidence for products that can aid the human body in breaking down and catabolizing the Spike protein. We found two compounds, nattokinase and bromelain. Both of which additionally have fibrinolytic properties which are advantageous in the prothrombotic milieu induced by the persistent Spike protein. Curcumin was added for its anti-inflammatory properties in the setting of post-COVID and vaccine patients. The main safety caveats are bleeding and allergic reactions, both of which are manageable. It is our experience that both nattokinase and bromelain can be used in addition to antiplatelet and anticoagulant drugs with physician monitoring. The empiric regimen can be continued for 3-12 months or more and be guided by clinical observation: -Nattokinase 2000 FU (100) mg orally twice a day without food -Bromelain 500 mg orally once a day without food -Curcumin 500 mg orally twice a day (nano, liposomal, or with piperine additive suggested) No therapeutic claims can be made for this regimen because it has not been tested in large, prospective, double-blind, placebo-controlled randomized trials. No such studies are planned or funded currently by federal or institutional sponsors. The main caveats are bleeding and allergic reactions. The regimen can be used in addition to antiplatelet and antithrombic agents; however, caution is advised with respect to monitoring bleeding risks. McCullough PA, Wynn C, Procter BC. Clinical Rationale for SARS-CoV-2 Base Spike Protein Detoxification in Post COVID-19 and Vaccine Injury Syndromes. Journal of American Physicians and Surgeons Volume 28 Number 3 Fall 2023, 90-93. Proteolytic Degradation of Spike Protein Nattokinase The spike protein has been found free, bound by antibodies, and also encased within lysosomes or exosomes both inside and outside of cells. Patterson et al. have found these, both after infection and after vaccination, likely worsened by repeated exposures (Figure 1). This shows that the spike protein can persist in the human body for a very long time (months to years), probably because it is resistant to proteolytic cleavage and disposal. Proteolytic cleavage of spike appears to be an important mechanism to initiate clearance of the protein by the reticuloendothelial system. Nattokinase is a naturally occurring proteolytic enzyme with thrombolytic properties derived from the fermentation of soy beans by Bacillus subtilis natto. The organism is a probiotic gram-positive spore-forming bacterium with veterinary and human applications. Nattokinase has been widely used as a cardiovascular supplement in Japan for its anti- atherosclerotic and antithrombotic properties. It has undergone safety testing in doses up to 80,000 fibrinolytic units (FU) daily. Kurosawa and colleagues have shown in humans that D-dimer concentrations at six and eight 8 hours, and blood fibrin/fibrinogen degradation products at four hours after administration of a single oral dose of 2,000 FU (100 mg) were elevated significantly (p < 0.05, respectively). Thus, an empiric starting dose could be 2,000 FU twice a day. Full pharmacokinetic and pharmacodynamic studies have not been completed, but several years of market use as an over-the-counter supplement suggests that nattokinase is safe, with the main caveat being excessive bleeding. Caution is needed with concurrent antiplatelet and anticoagulant drugs. Oba and colleagues performed a series of experiments with various concentrations of nattokinase in preclinical models. They found that nattokinase effectively stopped SARS-CoV-2 and bovine herpes virus type 1 infection of human cells in culture, and that the proteolytic effect of nattokinase was heat sensitive. Tanikawa et al. examined the effect of nattokinase on the spike protein of SARS-CoV-2. In the first experiment they demonstrated that spike was degraded in a time and dose-dependent manner in a cell lysate preparation that could be analogous to a vaccine recipient. The second experiment demonstrated that nattokinase degraded the spike protein in SARS-CoV-2-infected cells. This reproduced a similar study done by Oba and colleagues. Because of the risk of bleeding, patients must be strongly cautioned to seek medical supervision with combining this nutraceutical with concurrent antiplatelet and anticoagulant drugs. Additionally, allergic reactions can occur, especially in patients who have known soy allergies. There is insufficient information for the use of nattokinase in children or pregnant or lactating women. Bromelain Bromelain is a family of cysteine proteases, isolated from the pineapple stem (Ananas comosus). Traditionally, it has been used for its anti-inflammatory and healing effects in cases of arthritis and injury, while it has been approved in Europe for the debridement of burn wounds. Experimental studies have demonstrated that bromelain presents unique immunomodulatory actions: 1) downregulation of the proinflammatory prostaglandin PGE-2 through inhibition of NF-kB and cyclooxygenase 2 (COX-2); 2) upregulation of the antiinflammatory PGE-1 (Figure 1); 3) activation of inflammatory mediators (interleukin 1b, interleukin-6, tumor necrosis factor-a, and interferon-g) as an acute response to cellular stress, but also inhibition of inflammatory mediators in states of overt cytokine production; 4) modulation of T-cell responses in vitro and in vivo; and 5) enhancement of T-cell- dependent antigen-specific B-cell antibody responses. Importantly, bromelain exerts dose-dependent anticoagulant effects: 1) downregulation of PGE-2 and thromboxane A2 (TXA2), thus leading to relative excess of prostacyclin in platelets, and 2) promotion of fibrinolysis by stimulating the conversion of plasminogen to plasmin and prevention of platelet aggregation (Figure 1). Bromelain also hydrolyzes bradykinin and reduces kininogen and bradykinin levels in serum and tissues, improving inflammation and edema as shown in animal studies. Notably, the latter action supports a potential role of bromelain in alleviating COVID-19 symptoms such as cough, fever, and pain, and the more serious implications of inflammation, thrombosis, and edema. The effect of bromelain on PGE-2 inhibition exceeds that of prednisone and aspirin, presenting very low toxicity and no major side effects. Additionally, a recent experimental study demonstrated that bromelain inhibits infection of VeroE6 cells by SARS- CoV-2 through blocking the virus binding and entry into cells by downregulation of ACE-2 and TMPRSS2 expression, and cleavage of the SARS-CoV-2 spike protein, presenting a novel and promising therapeutic option, which warrants further investigation. Bromelain increases the prothrombin time and partial thromboplastin time and can thus increase bleeding risk. It can cause gastrointestinal upset. Severe allergic reactions can occur. Bromelain may increase the absorption of medications—including antibiotics (such as tetracycline and amoxicillin), chemotherapeutic agents (such as 5-fluorouracil and vincristine), ACE inhibitors (such as captopril and lisinopril), benzodiazepines, certain antidepressants, opioids, and barbiturates. Physician supervision is advised. A standard dose of bromelain for human use is 500 mg orally per day. Inhibition of Spike and Its Fragments in Tissues Curcumin Curcumin (diferuloylmethane) is derived from turmeric (Curcuma longa), a member of the ginger family of plants. Curcumin is a polyphenol and modulates inflammation in the setting of viral infections via inhibition of cytokines through multiple transcription factors. Additionally, curcumin inhibits angiotensin converting enzyme (ACE), modulating angiotensin II synthesis, and promotes fibrinolysis and the anticoagulation process (see Figures 1 and 2). The antiviral actions of curcumin against multiple viruses (influenza and hepatitis viruses, herpes viruses, human papilloma virus, human immunodeficiency virus, severe acute respiratory syndrome coronavirus and other coronaviruses), bacteria, and fungi have been suggested in prior mechanistic studies. In silico studies have demonstrated that curcumin prevents SARS-CoV-2 entry into cells by blocking the spike protein binding sites and the cell ligands (ACE-2 receptors and TMPRSS-2), and by that mechanism reduces viral replication. The minimal absorption of curcumin following oral administration has been overcome with nanoparticle technology. Randomized trials have consistently showed reductions in hs-CRP and other inflammatory markers in the setting of spike protein mediated infection/injury. The World Health Organization (WHO) has determined 0–3 mg per kilogram of body weight to be an acceptable daily dietary intake, about 250 mg. At higher therapeutic doses there can be gastrointestinal adverse events including peptic ulcer disease. Nano or liposomal curcumin is available as an oral supplement with better absorption dosed at 500 mg twice a day and has been shown to be safe without liver or serious gastrointestinal toxicity. Alternatively, curcumin can be combined with piperine (black pepper extract), at about 10 mg/1000 mg, to significantly increase absorption. There are, however, published studies showing that curcumin supplements decrease effectiveness of prescription hormones thyroid and estradiol, so patients on these prescription medicines need to be monitored by their physicians to avoid being destabilized by the addition of curcumin. The same caution applies to turmeric supplements. Download PDF: https://jpands.org/vol28no3/mccullough.pdf Source: Dr. Petermcculloughmd, Wikipedia Also Read: